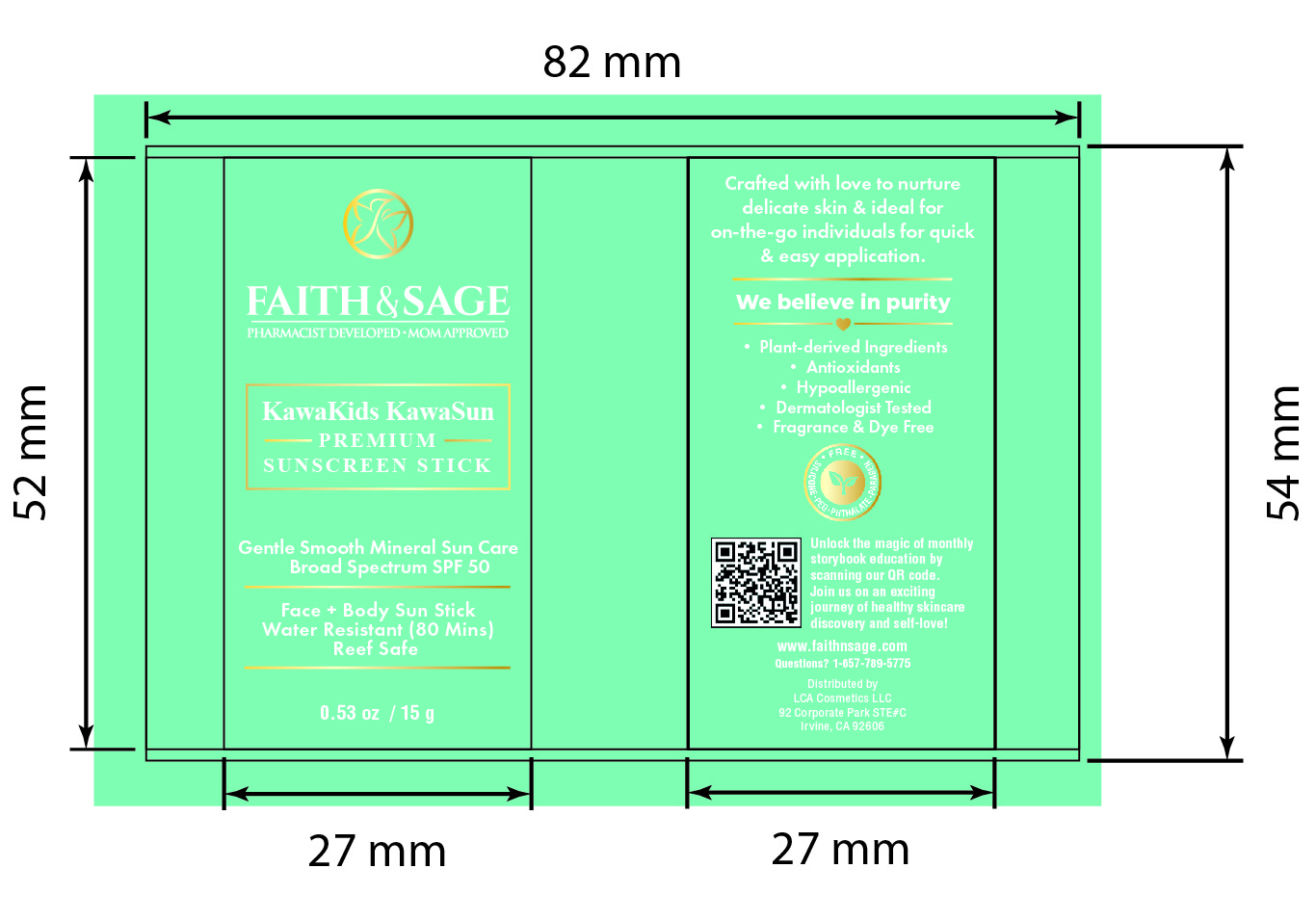
Label: KAWAKIDS KAWASUN - PREMIUM ON-THE-GO MINERAL SUNSCREEN SPF 50- zinc oxide stick
- NDC Code(s): 84569-911-15
- Packager: LCA COSMETICS LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated August 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
-
Directions
- Apply liberally to exposed areas 15 minutes before sun exposure
- Reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every two hours
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other protection measures including:
- Limit time in the sun, especially from 10am-2pm
- Wear long-sleeve shirts, pants, hats, and sunglasses
- Children under 6 months: ask a doctor
-
Inactive Ingredients
Persea Gratissima (Avocado) Oil, Caprylic/Capric Triglycerides, Butyrospermum Parkii (Shea) Butter, Olive Squalane (Suqalane), Cera Alba (Beeswax), Euphorbia Certifera (Candellia) Wax, Polyhydroxystearic Acid, Aloe Barbadensis (Aloe Vera) Leaf Extract, Piper Excelsum (Kawakawa) Extract, Simmondsia Chinensis (Jojoba) Esters, Calendula (Calendula) Officinalis Extract, Avena Sativa (Oat) Kernel Flour, Lactobacillus Ferment, Tamarindus Indica (Tamarind) Seed Extract
- Other Information
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
KAWAKIDS KAWASUN - PREMIUM ON-THE-GO MINERAL SUNSCREEN SPF 50
zinc oxide stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84569-911 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.25 g in 1 g Inactive Ingredients Ingredient Name Strength SQUALANE (UNII: GW89575KF9) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) AVOCADO OIL (UNII: 6VNO72PFC1) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) BUTYROSPERMUM PARKII (SHEA) BUTTER UNSAPONIFIABLES (UNII: 0C9AC7D6XU) WHITE WAX (UNII: 7G1J5DA97F) CANDELILLA WAX (UNII: WL0328HX19) ALOE VERA LEAF (UNII: ZY81Z83H0X) PIPER EXCELSUM LEAF (UNII: 0258808825) JOJOBA OIL (UNII: 724GKU717M) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) OATMEAL (UNII: 8PI54V663Y) LIMOSILACTOBACILLUS FERMENTUM (UNII: 2C1F12C6AP) TAMARIND SEED (UNII: 6AHP8A7OML) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84569-911-15 1 in 1 CARTON 08/09/2024 1 15 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/09/2024 Labeler - LCA COSMETICS LLC (058943126) Establishment Name Address ID/FEI Business Operations Spencer Specialty Products, Inc 084478941 manufacture(84569-911)