Label: NYSTATIN powder
- NDC Code(s): 67296-1501-3
- Packager: RedPharm Drug
- This is a repackaged label.
- Source NDC Code(s): 69315-306
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTIONNystatin is a polyene antifungal antibiotic obtained from - Streptomyces noursei. The molecular formula for Nystatin is C47H75NO17. The molecular weight of Nystatin is 926.1. Structural ...
-
CLINCAL PHARMACOLOGYPharmacokinetics - Nystatin is not absorbed from intact skin or mucous membrane. Microbiology - Nystatin is an antibiotic which is both fungistatic and fungicidal - in vitroagainst a wide ...
-
INDICATIONS AND USAGENystatin topical powder is indicated in the treatment of cutaneous or mucocutaneous mycotic infections caused by - Candida albicansand other susceptible - Candida species. Nystatin topical ...
-
CONTRAINDICATIONSNystatin topical powder is contraindicated in patients with a history of hypersensitivity to - anyof its components.
-
PRECAUTIONSGeneral - Nystatin topical powder should not be used for the treatment of systemic, oral, intravaginal or ophthalmic infections. If irritation or sensitization develops, treatment should be ...
-
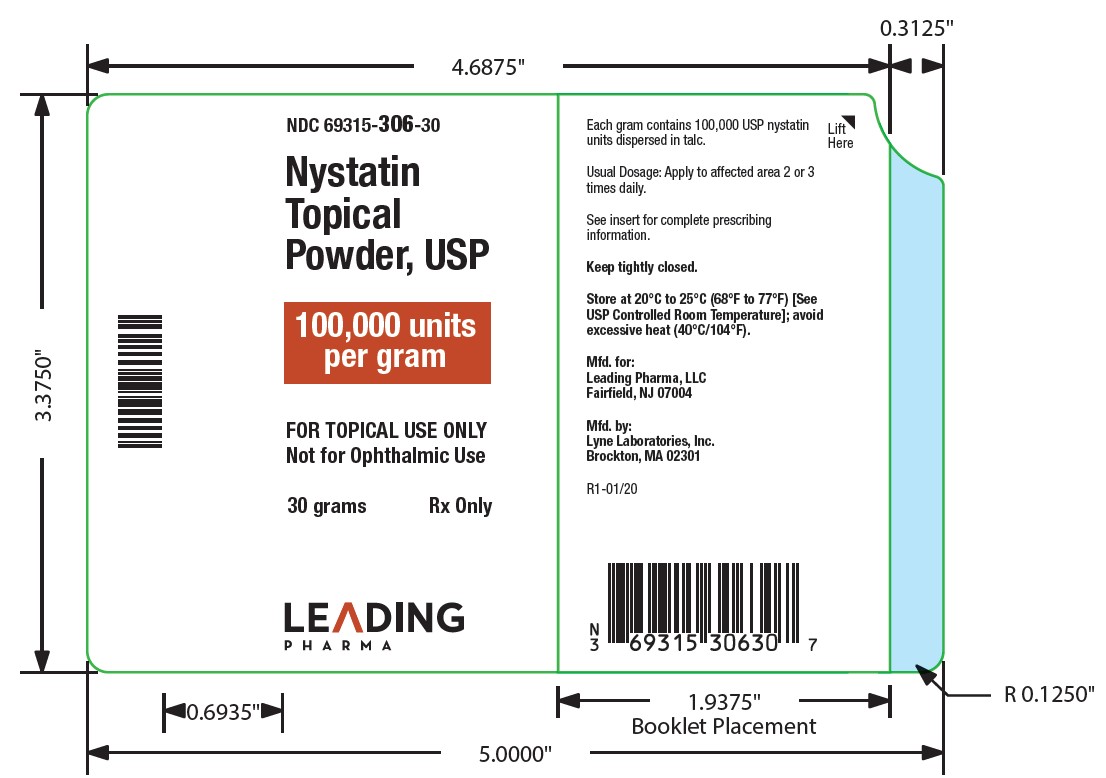
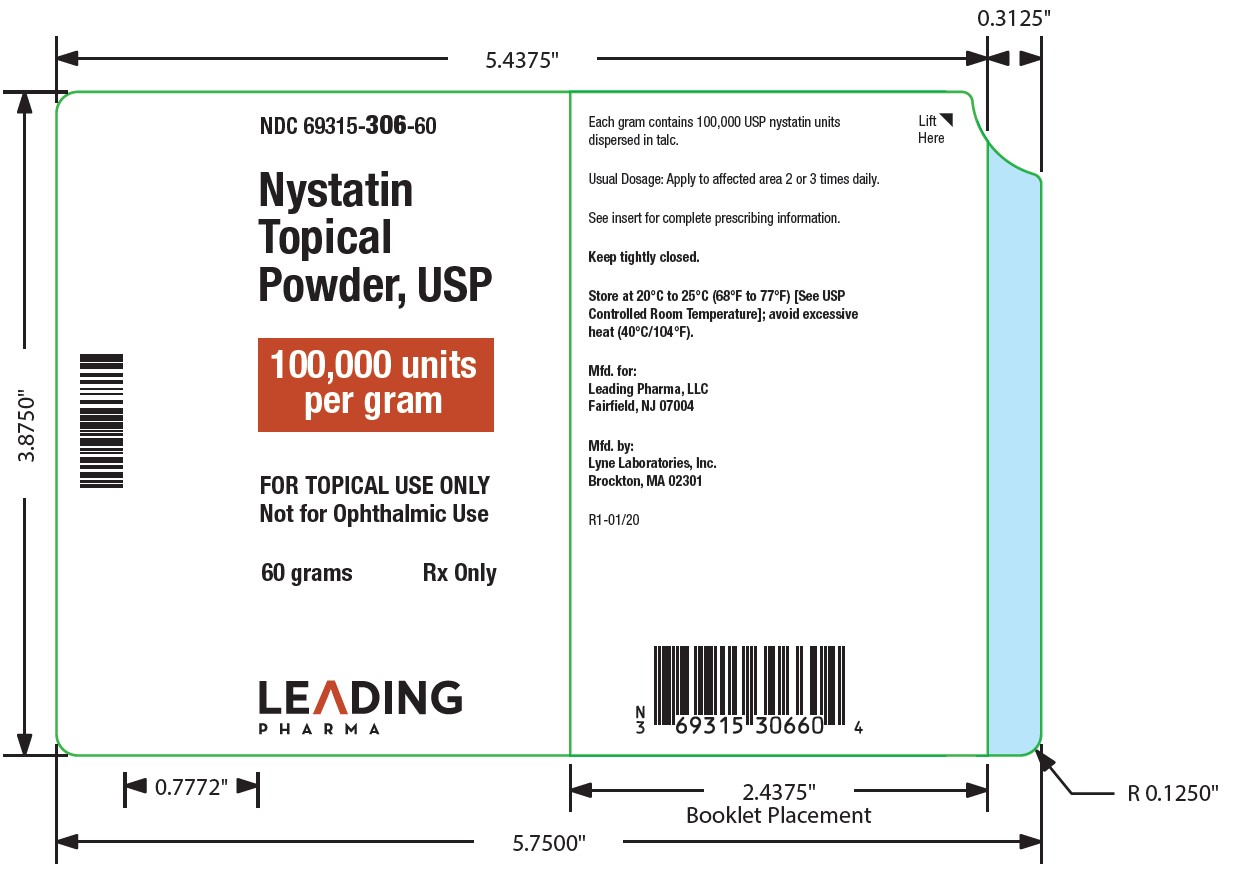
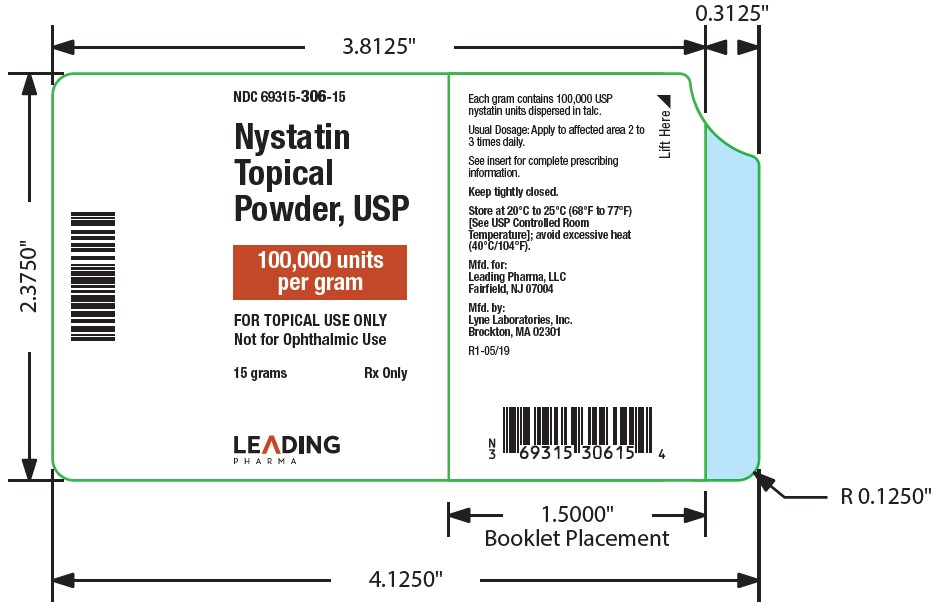
HOW SUPPLIEDNystatin topical powder is supplied as 100,000 units nystatin per gram in plastic squeeze bottles: 15g (NDC 69315-306-15) 30g (NDC 69315-306-30) 60g (NDC 69315-306-60) STORAGE - Store at ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information