Label: ZITHROMAX- azithromycin dihydrate tablet, film coated
- NDC Code(s): 67296-1694-6
- Packager: RedPharm Drug
- This is a repackaged label.
- Source NDC Code(s): 0069-4061
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZITHROMAX - ®safely and effectively. See full prescribing information for ZITHROMAX. ZITHROMAX (azithromycin) 250 mg and 500 mg ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEZITHROMAX (azithromycin) is a macrolide antibacterial drug indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms ...
-
2 DOSAGE AND ADMINISTRATION2.1 Adult Patients - [see - Indications and Usage (1.1)and - Clinical Pharmacology (12.3)] Infection*Recommended Dose/Duration of Therapy - * DUE TO THE INDICATED ...
-
3 DOSAGE FORMS AND STRENGTHSZITHROMAX 250 mg tablets are supplied as pink modified capsular shaped, engraved, film-coated tablets containing azithromycin dihydrate equivalent to 250 mg of azithromycin. ZITHROMAX 250 mg ...
-
4 CONTRAINDICATIONS4.1 Hypersensitivity - ZITHROMAX is contraindicated in patients with known hypersensitivity to azithromycin, erythromycin, any macrolide or ketolide drug. 4.2 Hepatic Dysfunction - ZITHROMAX is ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity - Serious allergic reactions, including angioedema, anaphylaxis, and dermatologic reactions including Acute Generalized Exanthematous Pustulosis (AGEP), Stevens-Johnson ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in labeling: Hypersensitivity - [see - Warnings and Precautions (5.1)] Hepatotoxicity - [see ...
-
7 DRUG INTERACTIONS7.1 Nelfinavir - Co-administration of nelfinavir at steady-state with a single oral dose of azithromycin resulted in increased azithromycin serum concentrations. Although a dose adjustment of ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from published literature and postmarketing experience over several decades with azithromycin use in pregnant women have not identified any ...
-
10 OVERDOSAGEAdverse reactions experienced at higher than recommended doses were similar to those seen at normal doses particularly nausea, diarrhea, and vomiting. In the event of overdosage, general ...
-
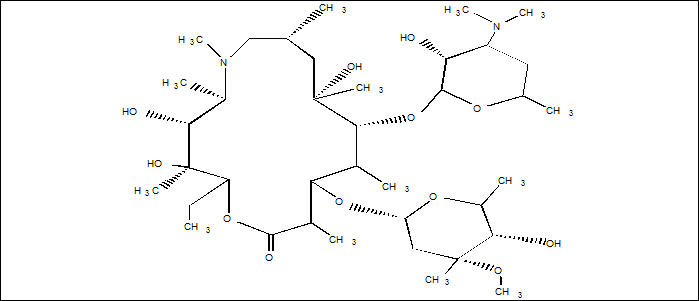
11 DESCRIPTIONZITHROMAX (azithromycin tablets and azithromycin for oral suspension) contain the active ingredient azithromycin, a macrolide antibacterial drug, for oral administration. Azithromycin has the ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Azithromycin is a macrolide antibacterial drug. [see - Microbiology (12.4)] 12.2 Pharmacodynamics - Based on animal models of infection, the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals have not been performed to evaluate carcinogenic potential. Azithromycin has shown no mutagenic potential ...
-
14 CLINICAL STUDIES14.1 Adult Patients - Acute Bacterial Exacerbations of Chronic Bronchitis - In a randomized, double-blind controlled clinical trial of acute exacerbation of chronic bronchitis (AECB) ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGZITHROMAX is supplied in the following strengths and package configurations: Tablet strengthTablet Color/ShapeTablet MarkingsPackage SizeNDC Code - 250 mg - (containing ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). General Patient Counseling - ZITHROMAX tablets and oral suspension can be taken with or without ...
-
Patient InformationZITHROMAX - ®(Zith-roe-maks) (azithromycin) Tablets - ZITHROMAX - ® (azithromycin) Oral Suspension - Read this Patient Information leaflet before you start taking ZITHROMAX ...
-
PRINCIPAL DISPLAY PANEL - 250 mg - 30 Tablet Bottle LabelNDC 0069-3060-30 - Pfizer - Zithromax® (azithromycin) tablets - 250 mg* 30 Tablets - Rx only
-
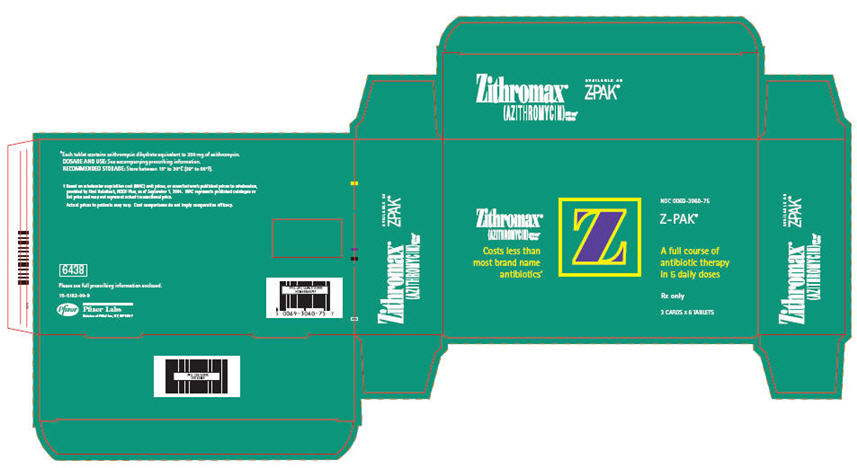
PRINCIPAL DISPLAY PANEL - 250 mg - 6 ct. Blister PackZithromax® (AZITHROMYCIN) 250 mg* Tablets - Costs less - than most - brand name - antibiotics - † Z - Z-PAK - ® A full course - of antibiotic - therapy ...
-
PRINCIPAL DISPLAY PANEL - 250 mg - 10 ct. Blister PackZithromax® (azithromycin) Tablet - 250 mg - Distributed by - PFIZER LABS - DIV OF PFIZER INC, NY, NY 10017 - MADE IN USA (includes foreign content) 13074600 - EXP & LOT AREA
-
PRINCIPAL DISPLAY PANEL - 250 mg - 18 Tablet Blister PackZithromax - ® (AZITHROMYCIN) 250 mg* Tablets - Costs less than - most brand name - antibiotics - † NDC 0069-3060-75 - Z-PAK - ® A full course of - antibiotic therapy ...
-
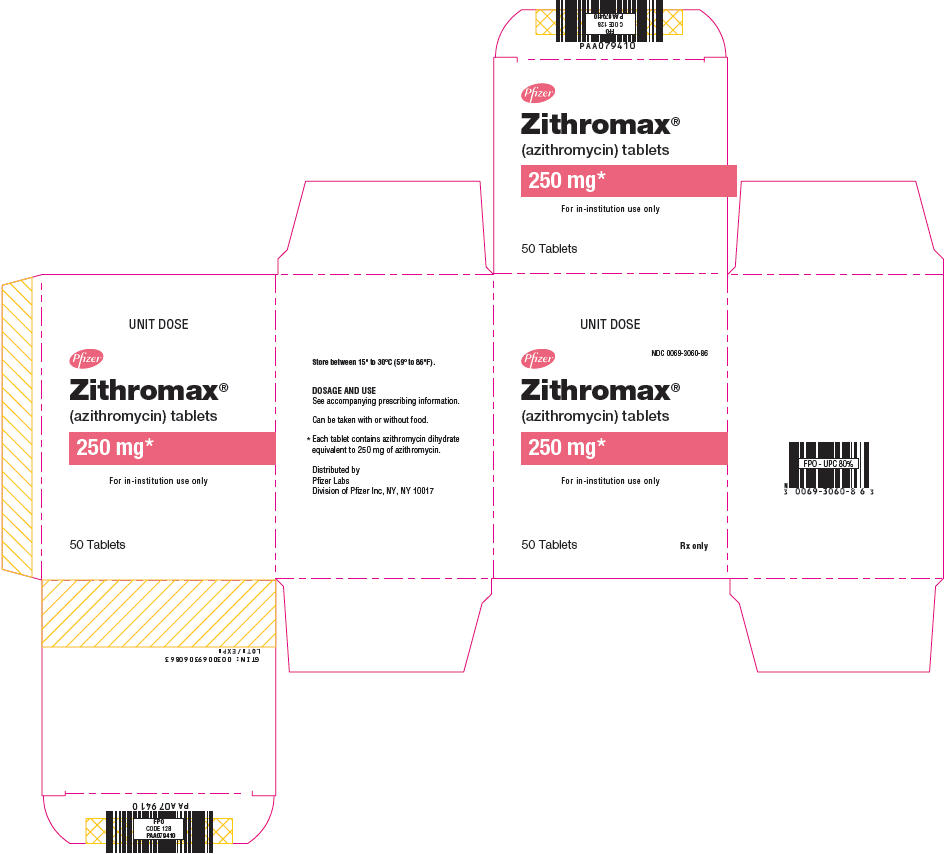
PRINCIPAL DISPLAY PANEL - 250 mg Tablet Blister Pack BoxUNIT DOSE - NDC 0069-3060-86 - Pfizer - Zithromax® (azithromycin) tablets - 250 mg* For in-institution use only - 50 Tablets - Rx only
-
PRINCIPAL DISPLAY PANEL - 500 mg - 30 Tablet Bottle LabelNDC 0069-3070-30 - Pfizer - Zithromax - ® (azithromycin) tablets - 500 mg* 30 Tablets - Rx only
-
PRINCIPAL DISPLAY PANEL - 500 mg - 3 ct. Tablet Blister PackRx only - Z - Zithromax® (AZITHROMYCIN)500 mg Tablets - TRI-PAK™ 3 tablets x 500 mg* A full course of - antibiotic therapy in - 3 DAILY DOSES
-
PRINCIPAL DISPLAY PANEL - 500 mg - 3 ct. Tablet Blister CartonNDC 0069-3070-75 - Z - Zithromax® (AZITHROMYCIN)500 mg Tablets - TRI-PAK™ 3 Cards x Three 500 mg* Tablets
-
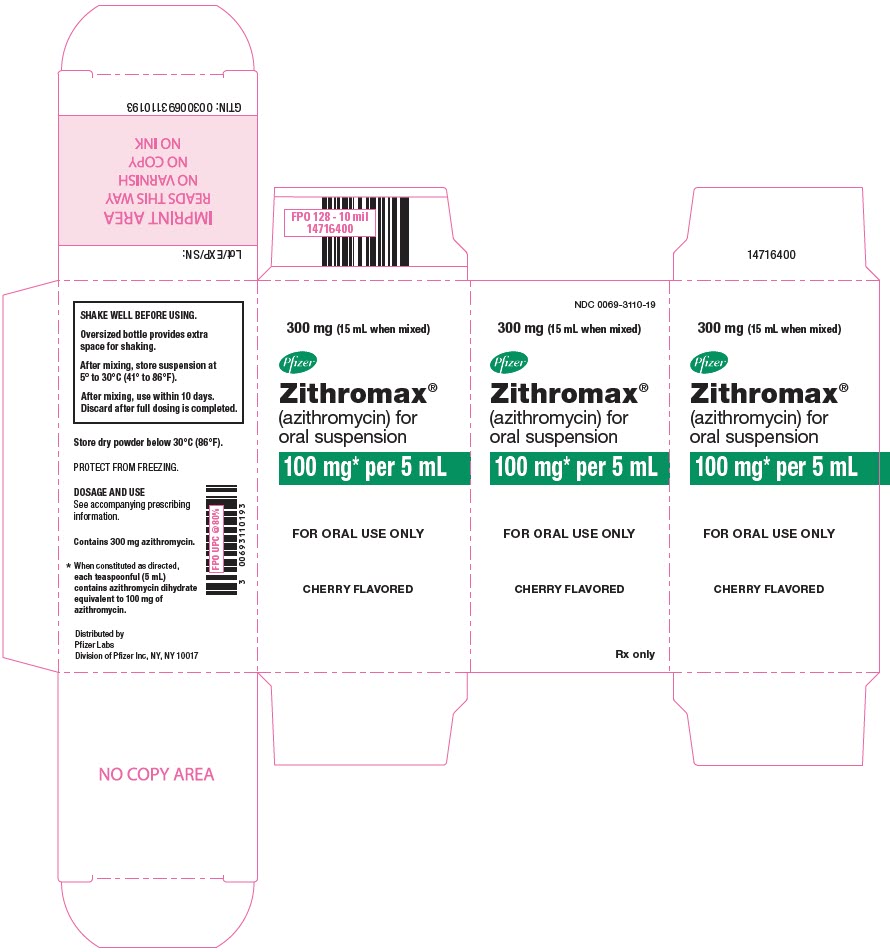
PRINCIPAL DISPLAY PANEL - 300 mg Bottle LabelNDC 0069-3110-19 - 300 mg (15 mL when mixed) Pfizer - Zithromax - ® (azithromycin) for - oral suspension - 100 mg* per 5 mL - CHERRY FLAVORED - Rx only
-
PRINCIPAL DISPLAY PANEL - 300 mg Bottle CartonNDC 0069-3110-19 - 300 mg (15 mL when mixed) Pfizer - Zithromax - ® (azithromycin) for - oral suspension - 100 mg* per 5 mL - FOR ORAL USE ONLY - CHERRY FLAVORED - Rx only
-
PRINCIPAL DISPLAY PANEL - 600 mg Bottle LabelNDC 0069-3120-19 - 600 mg (15 mL when mixed) Pfizer - Zithromax - ® (azithromycin) for - oral suspension - 200 mg* per 5 mL - CHERRY FLAVORED - Rx only
-
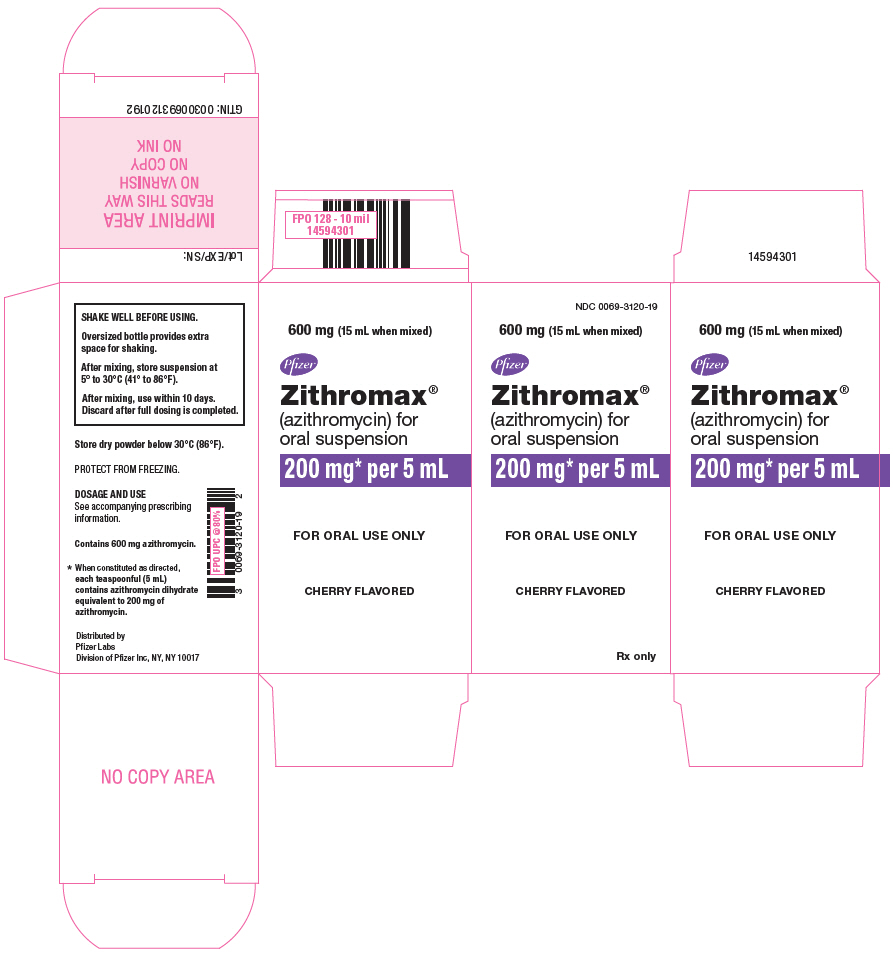
PRINCIPAL DISPLAY PANEL - 600 mg Bottle CartonNDC 0069-3120-19 - 600 mg (15 mL when mixed) www.zithromax.com - Pfizer - Zithromax - ® (azithromycin) for - oral suspension - 200 mg* per 5 mL - FOR ORAL USE ONLY - CHERRY FLAVORED - Rx only
-
PRINCIPAL DISPLAY PANEL - 900 mg Bottle LabelNDC 0069-3130-19 - 900 mg (22.5 mL when mixed) Pfizer - Zithromax - ® (azithromycin) for - oral suspension - 200 mg* per 5 mL - CHERRY FLAVORED - Rx only
-
PRINCIPAL DISPLAY PANEL - 900 mg Bottle CartonNDC 0069-3130-19 - 900 mg (22.5 mL when mixed) Pfizer - Zithromax - ® (azithromycin) for - oral suspension - 200 mg* per 5 mL - FOR ORAL USE ONLY - CHERRY FLAVORED - Rx only
-
PRINCIPAL DISPLAY PANEL - 1200 mg Bottle LabelNDC 0069-3140-19 - 1200 mg (30 mL when mixed) Pfizer - Zithromax - ® (azithromycin) for - oral suspension - 200 mg* per 5 mL - CHERRY FLAVORED - Rx only
-
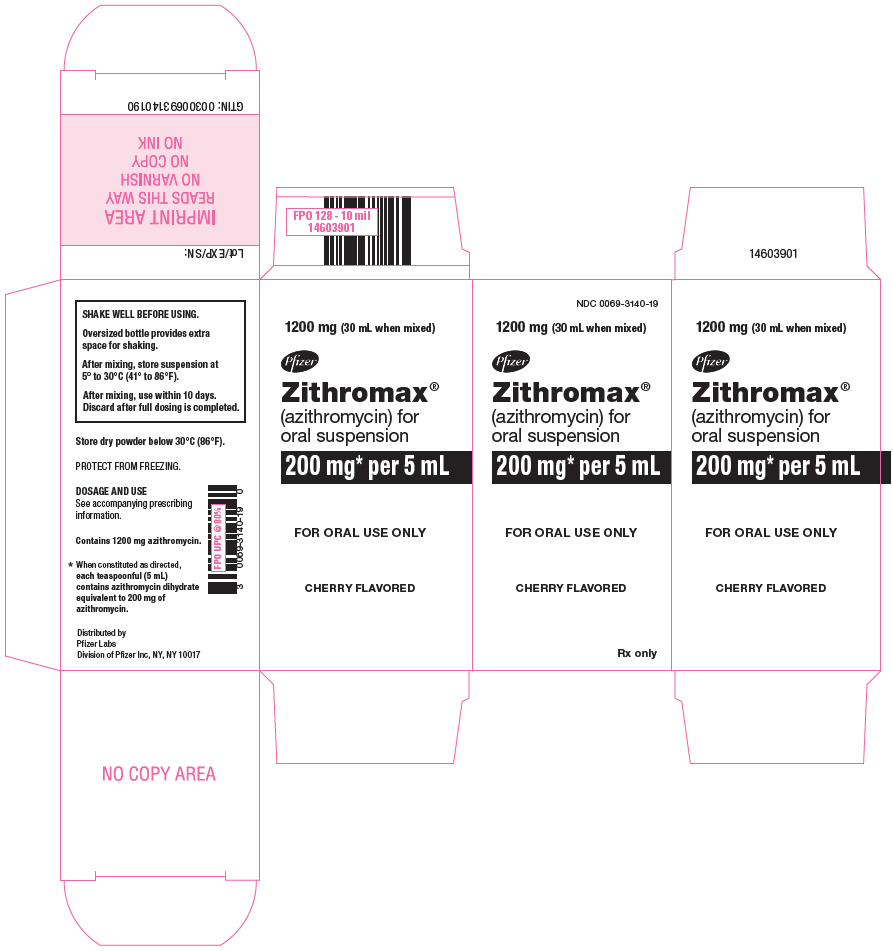
PRINCIPAL DISPLAY PANEL - 1200 mg Bottle CartonNDC 0069-3140-19 - 1200 mg (30 mL when mixed) Pfizer - Zithromax - ® (azithromycin) for - oral suspension - 200 mg* per 5 mL - FOR ORAL USE ONLY - CHERRY FLAVORED - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information