Label: ALBUTEROL SULFATE solution
- NDC Code(s): 67296-1698-1, 67296-1698-2
- Packager: RedPharm Drug
- This is a repackaged label.
- Source NDC Code(s): 0378-8270
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
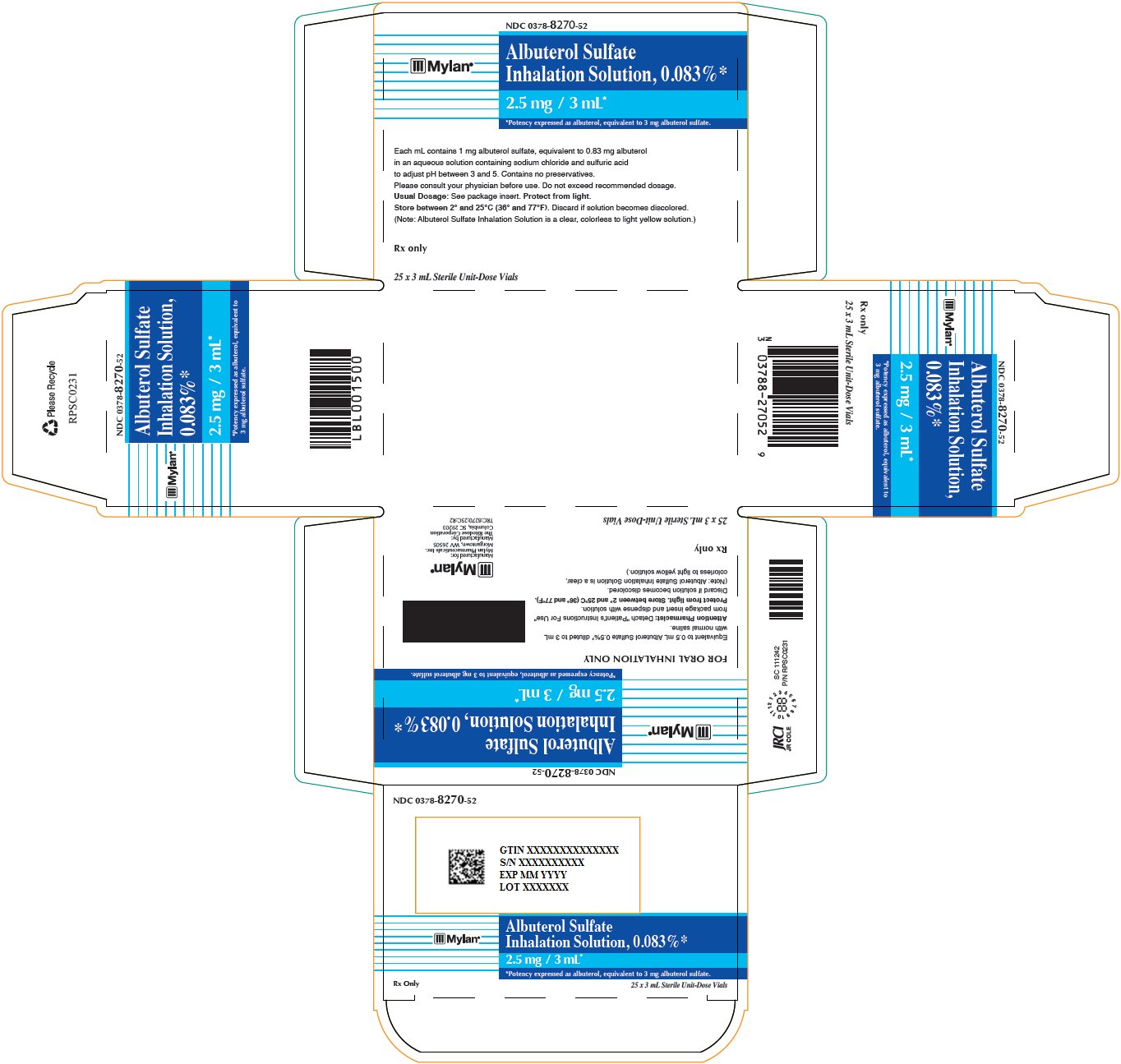
SPL UNCLASSIFIED SECTIONAlbuterol Sulfate Inhalation Solution 0.083% 1 - 1 - (Potency expressed as albuterol, equivalent to 3 mg albuterol sulfate)
-
SPL UNCLASSIFIED SECTIONPRESCRIBING INFORMATION - FOR INHALATION USE ONLY-NOT FOR INJECTION.
-
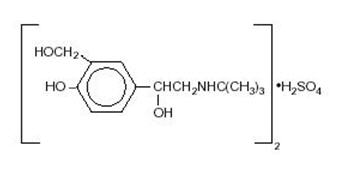
DESCRIPTIONAlbuterol sulfate inhalation solution is a relatively selective beta - 2-adrenergic bronchodilator (see - CLINICAL PHARMACOLOGYsection below). Albuterol sulfate, the racemic form of albuterol ...
-
CLINICAL PHARMACOLOGYThe prime action of beta-adrenergic drugs is to stimulate adenyl cyclase, the enzyme which catalyzes the formation of cyclic-3',5'-adenosine monophosphate (cyclic AMP) from adenosine triphosphate ...
-
INDICATIONS AND USAGEAlbuterol sulfate inhalation solution is indicated for the relief of bronchospasm in patients 2 years of age and older with reversible obstructive airway disease and acute attacks of ...
-
CONTRAINDICATIONSAlbuterol sulfate inhalation solution is contraindicated in patients with a history of hypersensitivity to any of its components.
-
WARNINGSAs with other inhaled beta-adrenergic agonists, albuterol sulfate inhalation solution can produce paradoxical bronchospasm, which can be life threatening. If it occurs, the preparation should be ...
-
PRECAUTIONSGeneral - Albuterol, as with all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias and ...
-
ADVERSE REACTIONSClinical Trial Experience - The results of clinical trials with albuterol sulfate inhalation solution in 135 patients showed the following side effects which were considered probably or possibly ...
-
OVERDOSAGEManifestations of overdosage may include seizures, anginal pain, hypertension, hypokalemia, tachycardia with rates up to 200 beats/min, and exaggeration of the pharmacological effects listed in ...
-
DOSAGE AND ADMINISTRATIONAdults and Children 2 to 12 Years of Age - The usual dosage for adults and for children weighing at least 15 kg is 2.5 mg of albuterol (one vial) administered three to four times daily by ...
-
HOW SUPPLIEDUnit-dose plastic vial containing Albuterol Sulfate Inhalation Solution 0.083%, 2.5 mg/3 mL - 2. Equivalent to 0.5 mL albuterol (as the sulfate) 0.5% (2.5 mg albuterol) diluted to 3 mL. Supplied ...
-
SPL UNCLASSIFIED SECTIONRx only. Manufactured for: Mylan Pharmaceuticals Inc. Morgantown, WV 26505 - Manufactured by: The Ritedose Corporation - Columbia, SC 29203 - TRC:ALSUIS:R3 - JAN 2013
-
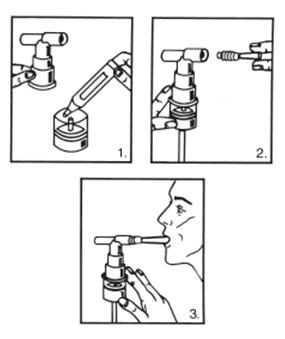
PATIENT'S INSTRUCTIONS FOR USEAlbuterol Sulfate Inhalation Solution 0.083%3 - Note: This is a unit-dose vial. No dilution is required. Read complete instructions carefully before using. Remove the vial from the foil ...
-
PRINCIPAL DISPLAY PANEL - 25 x 3 mL Vials CartonMylan - ® NDC 0378-8270-52 - Albuterol Sulfate - Inhalation Solution, 0.083%* 2.5 mg / 3 mL* *Potency expressed as albuterol, equivalent to 3 mg albuterol sulfate. FOR ORAL INHALATION ...
-
INGREDIENTS AND APPEARANCEProduct Information