Label: NEXGARD PLUS- afoxolaner, moxidectin and pyrantel tablet, chewable
- NDC Code(s): 0010-4260-01, 0010-4260-02, 0010-4260-03, 0010-4261-01, view more
- Packager: Boehringer Ingelheim Animal Health USA Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated June 3, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFor oral use in dogs only
-
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
-
Description:
NexGard® PLUS (afoxolaner, moxidectin, and pyrantel chewable tablets) is available in five sizes of beef-flavored, soft chewables for oral administration to dogs and puppies according to their ...
-
Indications: NexGard® PLUS is indicated for the prevention of heartworm disease caused by Dirofilaria immitis and for the treatment and control of adult hookworm (Ancylostoma caninum, Ancylostoma braziliense ...
-
Dosage and Administration: NexGard® PLUS is given orally once a month at the minimum dosage of 1.14 mg/lb (2.5 mg/kg) afoxolaner, 5.45 mcg/lb (12 mcg/kg) moxidectin, and 2.27 mg/lb (5.0 mg/kg) pyrantel (as pamoate salt) ...
-
Dosing Schedule: Body - Weight - (lbs.) Afoxolaner - Per - Chewable - (mg) Moxidectin - Per - Chewable - (mcg) Pyrantel* Per - Chewable - (mg) Chewables - Administered - 4 ...
-
Contraindications: There are no known contraindications for the use of NexGard® PLUS.
-
Warnings: Not for use in humans. Keep this and all drugs out of the reach of children. In case of accidental ingestion, contact a physician for treatment advice. Keep NexGard® PLUS in a secure location ...
-
Precautions: Afoxolaner, one of the ingredients in NexGard® PLUS, is a member of the isoxazoline class. This class has been associated with neurologic adverse reactions including tremors, ataxia, and ...
-
Adverse Reactions: In a field safety and effectiveness study, NexGard® PLUS was administered to dogs for the prevention of heartworm disease. The study included a total of 272 dogs (134 administered NexGard® PLUS ...
-
Contact Information: For a copy of the Safety Data Sheet (SDS) or to report suspected adverse drug events, contact Boehringer Ingelheim Animal Health USA Inc. at 1-888-637-4251 or www.nexgardforpets.com. For ...
-
Clinical Pharmacology: Mode of Action: NexGard® PLUS (afoxolaner, moxidectin, and pyrantel chewable tablets) contains the three active pharmaceutical ingredients afoxolaner, moxidectin, and pyrantel (as pamoate ...
-
Effectiveness: Heartworm Prevention: In two well-controlled laboratory studies, NexGard® PLUS was 100% effective against induced D. immitis infections when administered for six consecutive months. In a ...
-
Target Animal Safety: Margin of Safety: NexGard® PLUS was administered orally at 1, 3, and 5X the maximum exposure doses at approximately 28-day intervals for six treatments to 8-week-old Beagle puppies. Dogs in the ...
-
How Supplied: NexGard® PLUS is available in five strengths of beef-flavored soft chewables formulated according to the weight of the dog (see Dosage and Administration). Each chewable size is available in ...
-
Storage Information: Store in original package at or below 25°C (77°F) with excursions permitted up to 40°C (104°F).
-
SPL UNCLASSIFIED SECTIONApproved by FDA under NADA # 141-554 - Marketed by: Boehringer Ingelheim Animal Health USA Inc., Duluth, GA 30096 - NEXGARD® is a registered trademark of Boehringer Ingelheim Animal Health France ...
-
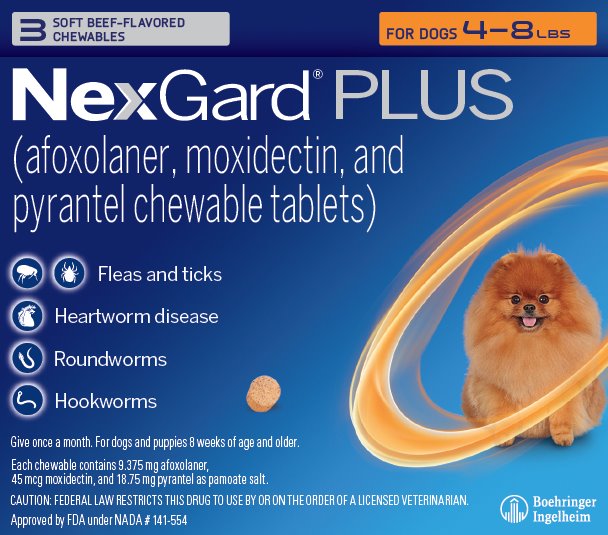
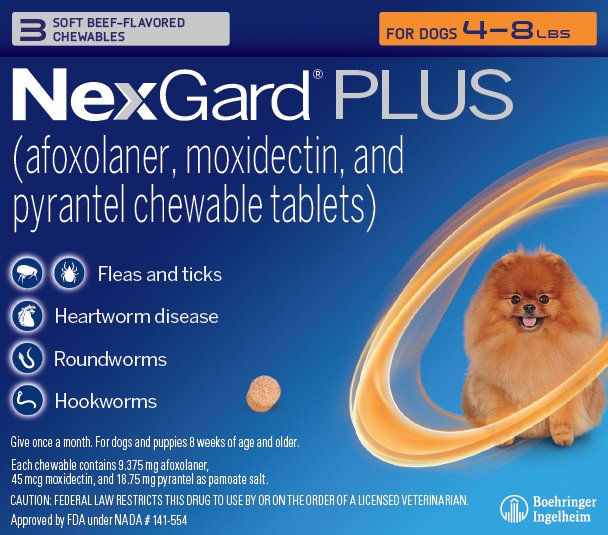
Principal Display Panel – Display Carton – For Dogs 4 – 8 lbs – 3 chewables
-
Principal Display Panel - Display Carton – For Dogs 8.1 - 17 lbs - 3 chewables

-
Principal Display Panel - Display Carton – For Dogs 17.1 - 33 lbs - 3 chewables

-
Principal Display Panel - Display Carton – For Dogs 33.1 – 66 lbs - 3 chewables

-
Principal Display Panel – Display Carton – For Dogs 66.1 – 132 lbs – 3 chewables

-
INGREDIENTS AND APPEARANCEProduct Information