LIVING A FULL LIFE WITH ASTHMA
-
PATIENT INSTRUCTIONS
-
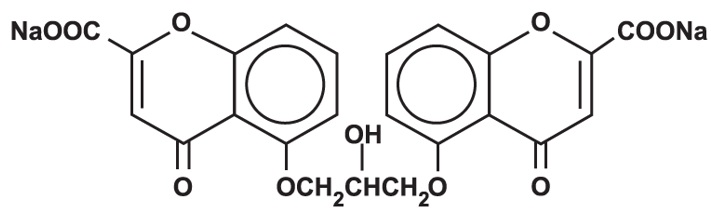
CROMOLYN SODIUM inhalation solution, USP
-
You or your child may be among the millions of Americans who have asthma. For most patients ...
LIVING A FULL LIFE WITH ASTHMA
PATIENT INSTRUCTIONS
CROMOLYN SODIUM inhalation solution, USP
You or your child may be among the millions of Americans who have asthma. For most patients, asthma need not limit your lifestyle, if you closely follow the asthma management plan your doctor provides you. Your doctor has given you this instruction sheet to help you learn more about asthma and ways to control it.
WHAT IS ASTHMA?
Asthma is a disease that causes patients to have difficulty breathing. Asthma "attacks" occur when the air passages (airways) to the lungs close up, blocking air from passing through. The closing up is caused by two things:
- The muscles around the airways tighten (constrict) making the airway narrower, and
- The passage lining swells and produces larger amounts of mucus (a sticky liquid normally found in airways). This swelling is caused by a certain type of inflammation that can build up in the airways of patients with asthma. Inflammation and the airway sensitivity that results from it cause further attacks to occur.
WHAT CAUSES THESE ATTACKS?
Asthma experts know that patients with asthma have attacks because their airways are inflamed and over reactive. Their lungs become super-sensitive to certain irritants or "triggers", such as cold dry air, pollen, smoke, or cat dander. In the presence of such triggers, someone with asthma may have an attack, and attacks may occur more often. Asthma triggers fall into 6 categories:
- Substances that cause allergies (pollens, animal dander, molds, house dust, some foods and medicines)
- Infections that affect breathing (colds, flu)
- Emotional stress (difficult situations at home, school, or work)
- Strenuous exercise
- Irritating gases (chlorine, perfume, tobacco smoke)
- Sudden changes in temperature or humidity
HOW TD PREVENT ASTHMA ATTACKS
No medicine or procedure will "cure" asthma. The key to asthma relief, therefore, is to prevent attacks and to relieve attack symptoms if they do occur.
A successful prevention plan* will do the following:
- Keep your activities, including exercise, at normal levels.
- Keep your lungs functioning normally or at a near-normal level.
- Prevent symptoms such as coughing or breathlessness that can keep you up at night, or occur in the early morning hours, or after exertion.
- Prevent asthma attacks from happening.
- Avoid unpleasant or harmful side effects that may result from using asthma medicine.
The best way to prevent asthma attacks is to avoid the triggers that bother you or your child. Try to identify the specific things that cause problems for you - things such as certain foods, house dust, or animal dander. Avoiding these triggers may be difficult or unpleasant. You may need to find a new home for a family pet or remove a carpet or a favorite stuffed toy. Even though these steps are difficult, they may be necessary in order to help prevent asthma attacks.
If house dust is a trigger, remove dust-collectors such as feather pillows, mattresses, quilts, and carpets from the bedroom. Mattresses and pillows can be covered with allergen-free covers. If a certain food triggers attacks, keep it out of your diet. Irritants in the air can be reduced by air conditioning, electrostatic air filters, or small-pore (HEPA) filters. Be careful of certain medicines. Aspirin and aspirin-like pain relievers, for example, can trigger attacks in some people and should be avoided if they do.
*Adapted from the National Heart, Lung, and Blood Institute: Guidelines for the Diagnosis and Management of Asthma, National Asthma Education Program, Expert Panel Report,1991.
OTHER DO'S AND DON'TS
Anyone with asthma should stick to a healthy, balanced diet, get lots of rest and moderate exercise, and follow these do's and dont's:
- Don't smoke, and don’t stay in the same room with people that do.
- Avoid fresh paint.
- Avoid sudden changes of temperature Don't go in and out of extremely cool air-conditioned buildings during hot weather.
- Stay home in extremely cold weather, if possible.
- Stay away from people with cold or flu.
- Try to avoid emotionally upsetting situations.
- Drink lots of liquids.
- Don't overdo, but follow a regular exercise plan, including activities that help develop lung capacity.
- Don't take any medicine on your own without asking your doctor first.
- Take all medicines your doctor prescribes, as much and as often as you are told.
- Avoid taking sleeping pills or sedatives, even if asthma keeps you awake. Prop yourself up with extra pillows until your asthma medicine takes effect.
- Avoid breathing in insecticides, deodorants, cleaning fluids, chlorine, or other irritating gases.
ASTHMA MEDICINES
Preventive Medicine
Your doctor knows that, besides relieving an attack when it happens, it is also important to prevent attacks from occurring in the first place. Therefore, he or she has prescribed for you cromolyn sodium, a medicine that prevents asthma attacks by making airways less sensitive to asthma triggers. It works by stabilizing cells in the airway lining called mast cells. During an asthma attack, these cells become unstable and give off chemicals called mediators that cause inflammation and asthma attacks.
By preventing mediator release, cromolyn sodium works to prevent asthma attacks.
Bronchodilators
When someone is having an asthma attack, he or she needs a medicine called a bronchodilator to open up (dilate) the blocked airways in order to relieve asthma attacks. Your doctor may have already prescribed this medicine for you to use at that time.
HOW WILL CROMOLYN SODIUM WORK FOR YOU?
To get the best possible results, follow your doctor's instructions carefully when you first take cromolyn sodium. Your doctor may tell you to take cromolyn sodium 10 to 15 minutes before you exercise or come into contact with a specific trigger, such as a cat. Usually, however, you will be told to take cromolyn sodium on a regular basis, probably starting at four times a day. It is crucial that you take cromolyn sodium, regularly, as otten as your doctor recommends, even though you have no asthma symptoms at the time. Cromolyn sodium starts working right away but when you first begin taking it, you may have a lot of inflammation in your airways. Therefore, it may take up to two weeks (or perhaps one month) of regular treatment to bring your asthma under control. Do not stop taking cromolyn sodium or skip any doses without first talking with your doctor.
When you start using cromolyn sodium for the first time, your doctor may ask you to keep a diary showing when you have any symptoms, if and when you have trouble sleeping, how often you wheeze or cough, and other notes to help determine how effective cromolyn sodium will be to help you prevent asthma attacks. Your doctor may also recommend the use of a peak flow meter daily to help you better assess your progress.
While taking cromolyn sodium on a regular basis, you may need to take a bronchodilator-type medicine to treat occasional symptoms or attacks. While taking cromolyn sodium, you should continue taking your other medications until your doctor advises you otherwise.
HOW TO TAKE CROMOLYN SODIUM
Be sure to follow instructions carefully when you are shown how to take cromolyn sodium inhalation solution.
CARE AND STORAGE
Cromolyn Sodium inhalation solution, USP should be stored at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Retain in foil pouch until time of use.
PROTECT FROM LIGHT.
Do not use if it contains a precipitate (particles or cloudiness) or becomes discolored.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
NOTE: In case of difficulty consult your doctor or pharmacist
Instructions for the Use of
Cromolyn sodium inhalation solution, USP
An aqueous solution for nebulization
NOT FOR INJECTION
For best results, follow these instructions exactly and observe Care and Storage directions.
METHOD OF ADMINISTRATION
Cromolyn sodium inhalation solution is recommended for use in a power driven nebulizer operated at an airflow rate of 6-8 liters per minute and equipped with a suitable face mask. Hand operated nebulizers are not suitable for the administration of cromolyn sodium inhalation solution. Your doctor will advise on the choice of a suitable nebulizer and how it should be used. Do not use any appliance without consulting your doctor.
Drug stability and safety of cromolyn sodium inhalation solution when mixed with other drugs in a nebulizer have not been established.
DOSAGE
Nebulization should be carried out four times a day at regular intervals, or as directed by your doctor. Use the contents of a fresh vial each time.
INHALATION
Once the nebulizer has been assembled and contains cromolyn sodium inhalation solution, hold the mask close to the patient's face and switch on the device. The patient should breathe in through the mouth and out through the nose in a normal, relaxed manner. Nebulization should take approximately five to ten minutes.
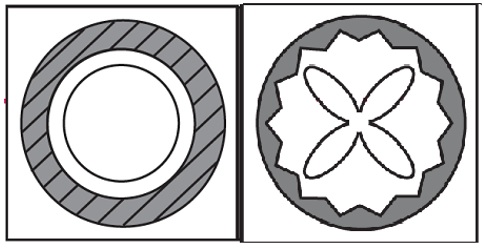
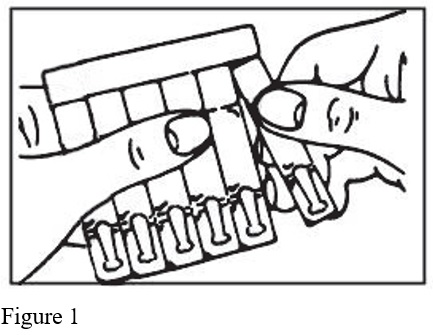
- Remove a single unit-dose vial from strip (Figure 1).
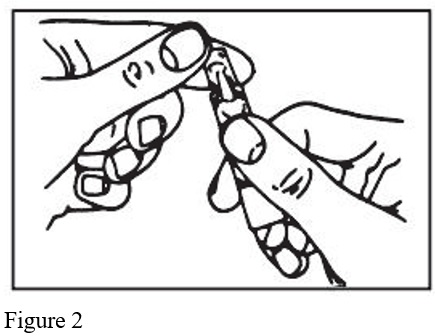
- Open the unit-dose vial by twisting off the tabbed top section (Figure 2).
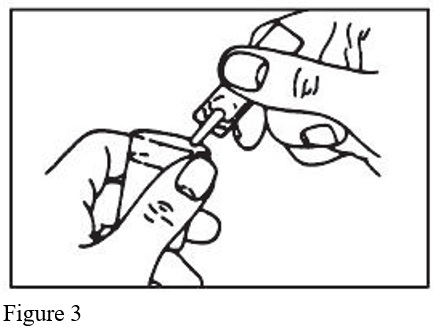
- Squeeze the contents of the unit-dose vial into the solution container of your nebulizer (Figure 3).
Discard the empty unit-dose vial.
Manufactured in Germany
for: Virtus Pharmaceuticals, LLC
VIRTUS®
PHARMACEUTICALS
Langhorne, PA 19047
Close