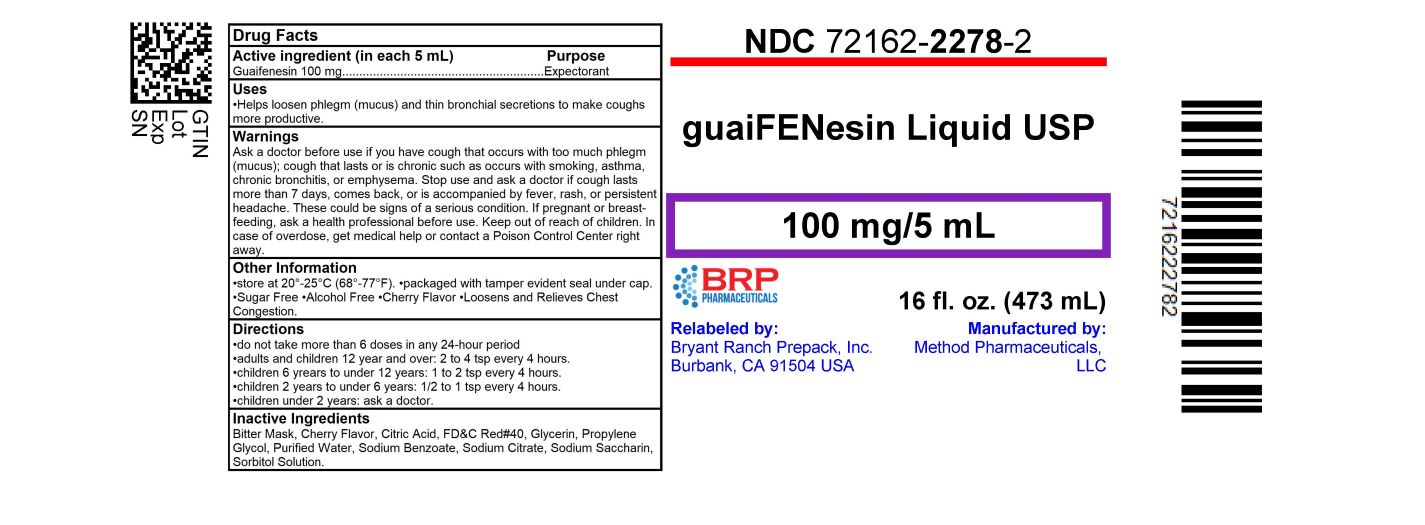
Label: GUAIFENESIN liquid
- NDC Code(s): 72162-2278-2
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 58657-508
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each 5 mL)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- Directions
- Other information
- Inactive ingredients
- Questions or Comments
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN
guaifenesin liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72162-2278(NDC:58657-508) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) CARAMEL (UNII: T9D99G2B1R) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SACCHARIN SODIUM (UNII: SB8ZUX40TY) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) Product Characteristics Color red Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72162-2278-2 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/22/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 04/01/2024 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(72162-2278) , RELABEL(72162-2278)