Label: NITROGLYCERIN tablet
- NDC Code(s): 67296-1775-1
- Packager: RedPharm Drug
- This is a repackaged label.
- Source NDC Code(s): 43598-437
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
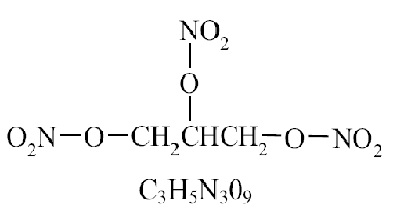
DESCRIPTIONNitroglycerin is a stabilized sublingual compressed nitroglycerin tablet that contains 0.3 mg, 0.4 mg, or 0.6 mg nitroglycerin USP; as well as calcium stearate powder, colloidal silicon dioxide ...
-
CLINICAL PHARMACOLOGYThe principal pharmacological action of nitroglycerin is relaxation of vascular smooth muscle. Although venous effects predominate, nitroglycerin produces, in a dose-related manner, dilation of ...
-
INDICATIONS AND USAGENitroglycerin sublingual tablets are indicated for the acute relief of an attack or acute prophylaxis of angina pectoris due to coronary artery disease.
-
CONTRAINDICATIONSNitroglycerin sublingual tablets are contraindicated in patients who are allergic to it. Sublingual nitroglycerin therapy is contraindicated in patients with early myocardial infarction, severe ...
-
WARNINGSThe benefits of sublingual nitroglycerin in patients with acute myocardial infarction or congestive heart failure have not been established. If one elects to use nitroglycerin in these conditions ...
-
PRECAUTIONSGeneral - Only the smallest dose required for effective relief of the acute anginal attack should be used. Excessive use may lead to the development of tolerance. Nitroglycerin sublingual tablets ...
-
ADVERSE REACTIONSHeadache that may be severe and persistent may occur immediately after use. Vertigo, dizziness, weakness, palpitation, and other manifestations of postural hypotension may develop occasionally ...
-
OVERDOSAGEHemodynamic Effects: The effects of nitroglycerin overdose are generally the results of nitroglycerin’s capacity to induce vasodilatation, venous pooling, reduced cardiac output, and hypotension ...
-
DOSAGE AND ADMINISTRATIONOne tablet should be dissolved under the tongue or in the buccal pouch at the first sign of an acute anginal attack. The dose may be repeated approximately every 5 minutes until relief is ...
-
HOW SUPPLIEDNitroglycerin sublingual tablets are supplied in 3 strengths (0.3 mg, 0.4 mg, and 0.6 mg) in bottles containing 100 tablets each, with color-coded labels, and in color-coded Patient Convenience ...
-
PATIENT PACKAGE INSERTNitroglycerin Sublingual Tablets, USP - (nahy-truh-glis-er-in) Read this information carefully before you start - nitroglycerin sublingual tabletsand each time you refill your prescription ...
-
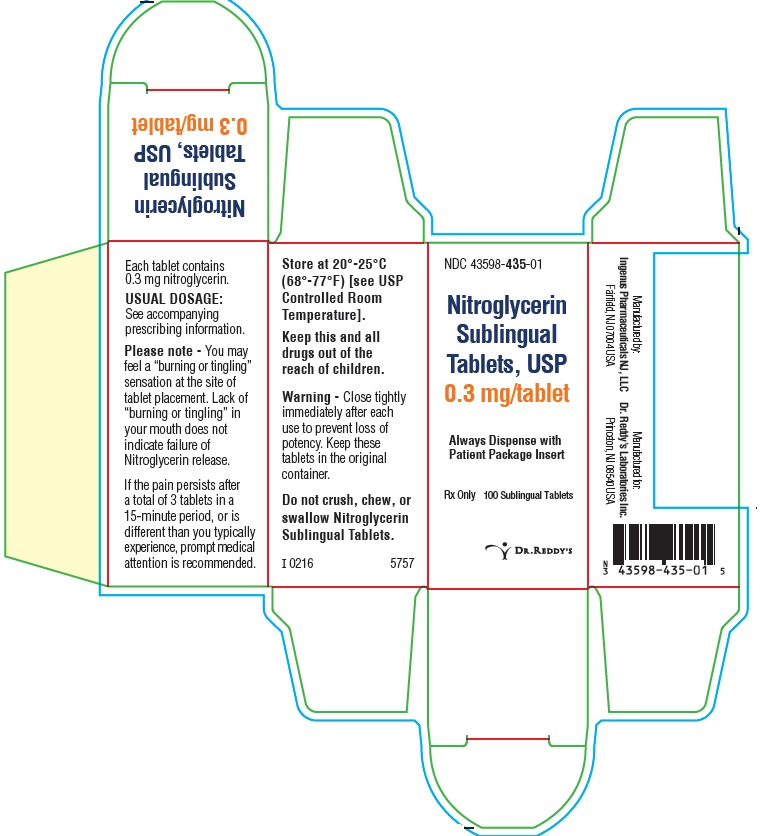
PACKAGE LABEL PRINCIPAL DISPLAY PANEL SECTION0.3 mg : Container Label: 100's
-
PRINCIPAL DISPLAY PANEL0.3 mg: Container Carton Label: 100's
-
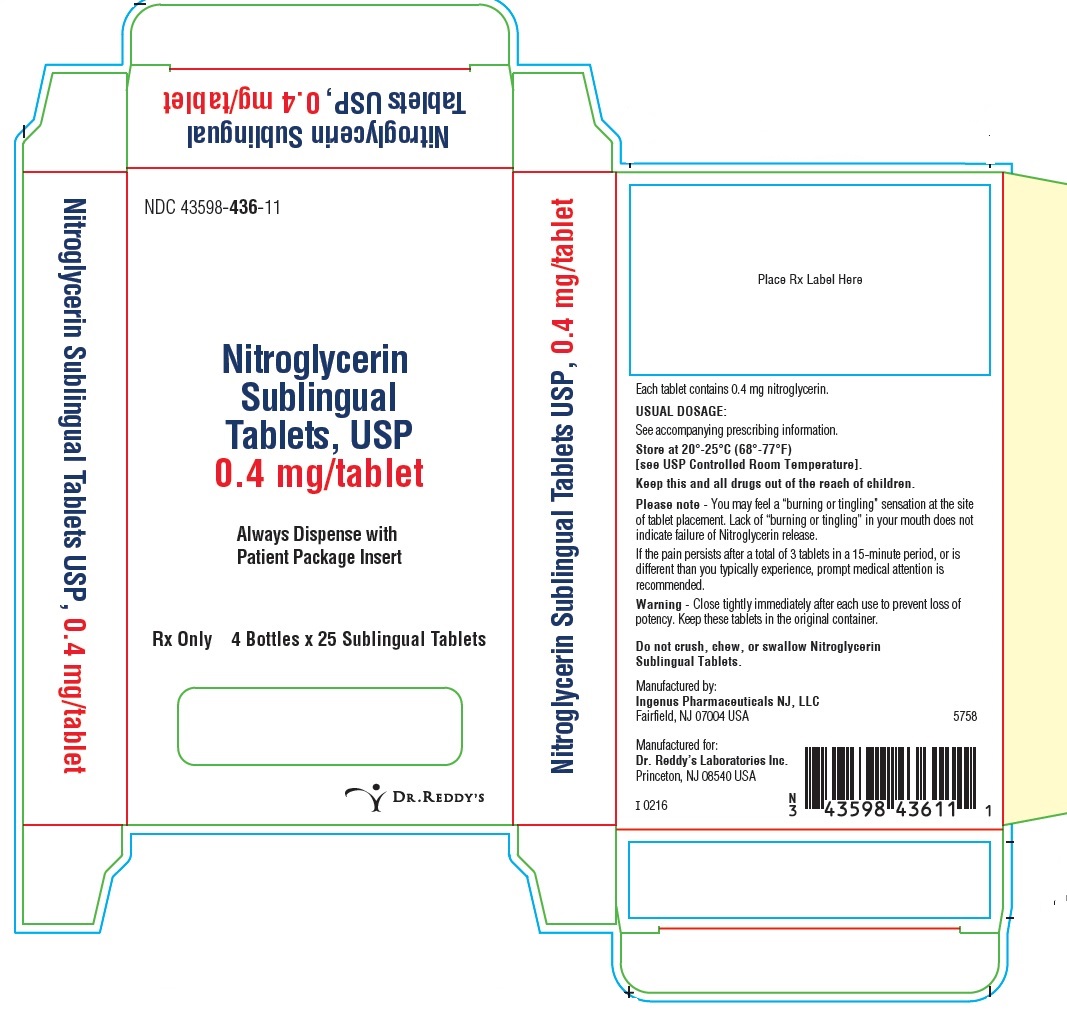
PRINCIPAL DISPLAY PANEL0.4 mg: Container Label: 25's
-
PRINCIPAL DISPLAY PANEL0.4 mg : Container Carton Label: 4 x 25's count
-
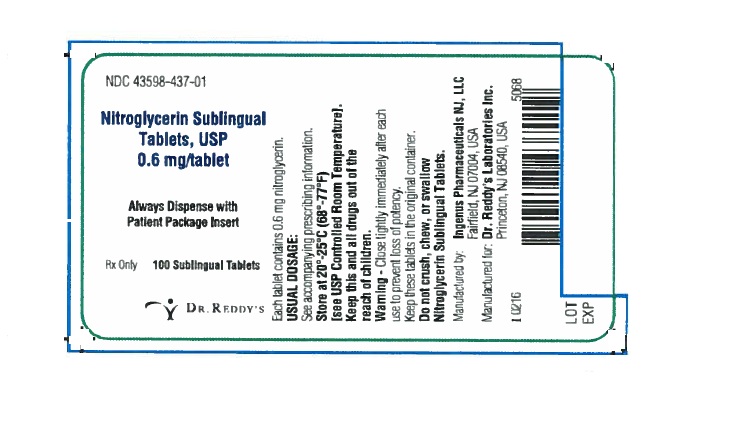
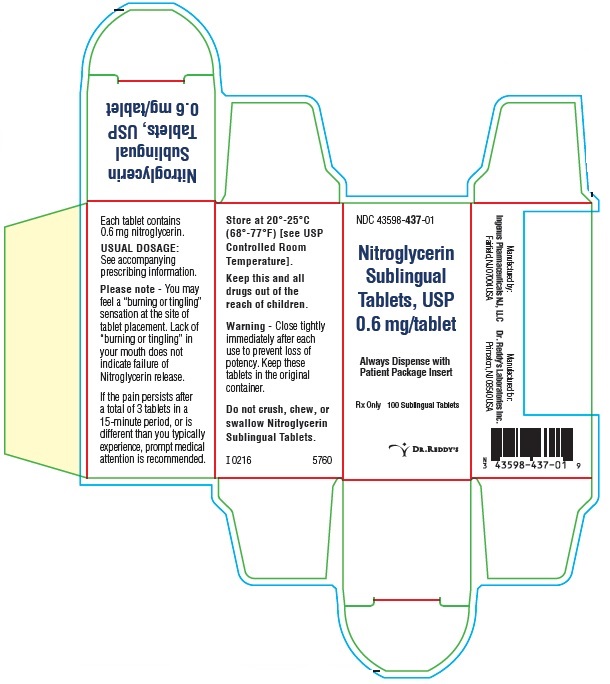
PRINCIPAL DISPLAY PANEL0.6 mg : Container Label: 100's
-
PRINCIPAL DISPLAY PANEL0.6 mg: Container Carton Label: 100's
-
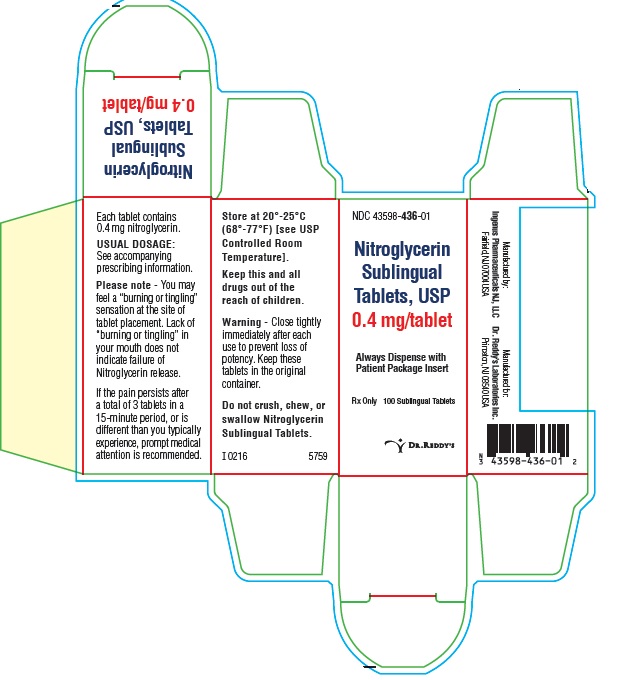
PRINCIPAL DISPLAY PANEL0.4 mg: Container Label : 100's
-
PRINCIPAL DISPLAY PANEL0.4 mg : Container Carton Label: 100's
-
INGREDIENTS AND APPEARANCEProduct Information