Label: METRONIDAZOLE gel
- NDC Code(s): 67296-2087-7
- Packager: RedPharm Drug
- This is a repackaged label.
- Source NDC Code(s): 0713-0575
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only - FOR INTRAVAGINAL USE ONLY. NOT FOR OPHTHALMIC, DERMAL, OR ORAL USE.
-
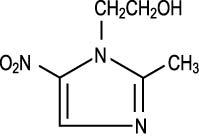
DESCRIPTIONMetronidazole vaginal gel is the intravaginal dosage form of the synthetic antibacterial agent, metronidazole, USP at a concentration of 0.75%. Metronidazole is a member of the imidazole class of ...
-
CLINICAL PHARMACOLOGYNormal Subjects - Following a single, intravaginal 5 grams dose of metronidazole vaginal gel (equivalent to 37.5 mg of metronidazole) to 12 normal subjects, a mean maximum serum metronidazole ...
-
INDICATIONS AND USAGEMetronidazole vaginal gel is indicated in the treatment of bacterial vaginosis (formerly referred to as - Haemophilusvaginitis, Gardnerellavaginitis, nonspecific vaginitis ...
-
CONTRAINDICATIONSMetronidazole vaginal gel is contraindicated in patients with a prior history of hypersensitivity to metronidazole, parabens, other ingredients of the formulation, or other nitroimidazole ...
-
WARNINGSConvulsive Seizures and Peripheral Neuropathy - Convulsive seizures and peripheral neuropathy, the latter characterized mainly by numbness or paresthesia of an extremity, have been reported in ...
-
PRECAUTIONSMetronidazole vaginal gel affords minimal peak serum levels and systemic exposure (AUCs) of metronidazole compared to 500 mg oral metronidazole dosing. Although these lower levels of exposure are ...
-
ADVERSE EVENTSClinical Trials - There were no deaths or serious adverse events related to drug therapy in clinical trials involving 800 non-pregnant women who received metronidazole vaginal gel. In a ...
-
OVERDOSAGEThere is no human experience with overdosage of metronidazole vaginal gel. Vaginally applied metronidazole gel, 0.75% could be absorbed in sufficient amounts to produce systemic effects. (See ...
-
DOSAGE AND ADMINISTRATIONThe recommended dose is one applicator full of metronidazole vaginal gel (approximately 5 grams containing approximately 37.5 mg of metronidazole) intravaginally once or twice a day for 5 days ...
-
HOW SUPPLIEDMetronidazole vaginal gel, 0.75% is supplied in a 70 gram tube and packaged with 5 vaginal applicators. The NDC number for the 70 gram tube is 0713-0575-71. Storage: Store at 20°C to 25°C (68°F to ...
-
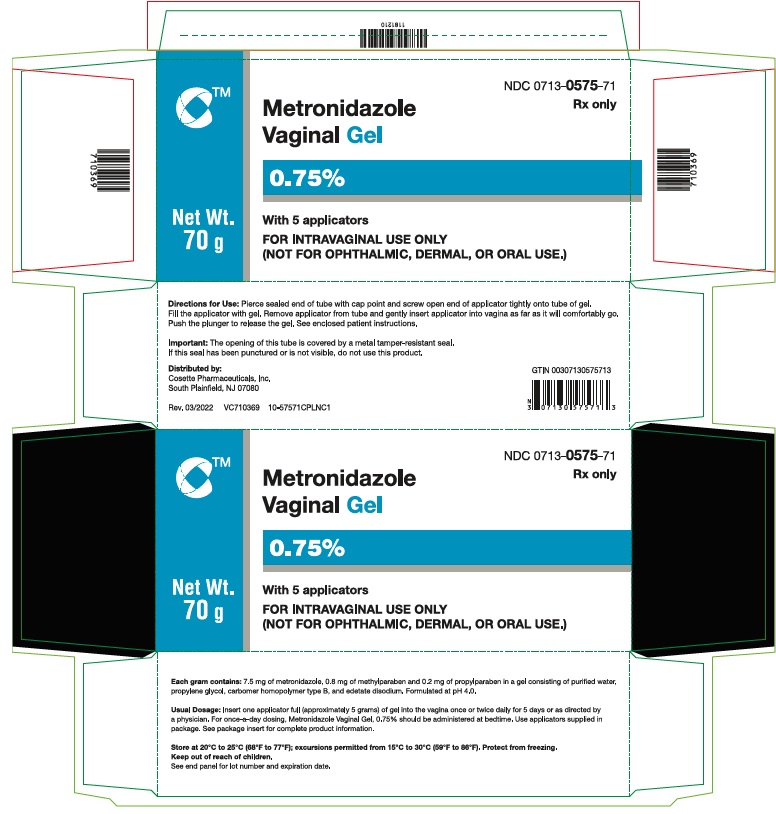
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 0713- 0575-71 - Rx only - METRONIDAZOLE - VAGINAL GEL, 0.75% with 5 applicators - FOR INTRAVAGINAL USE ONLY - (NOT FOR OPHTHALMIC, DERMAL, OR ORAL USE.) Net Wt. 70 g - Cosette ...
-
INGREDIENTS AND APPEARANCEProduct Information