Label: BETAMETHASONE VALERATE ointment
- NDC Code(s): 67296-2080-1
- Packager: RedPharm Drug
- This is a repackaged label.
- Source NDC Code(s): 0713-0327
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
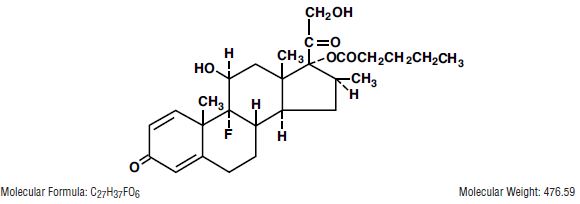
DESCRIPTIONBetamethasone Valerate Cream and Ointment contain betamethasone valerate USP, a synthetic adrenocorticosteroid for dermatologic use. Betamethasone, an analog of prednisolone, has a high degree of ...
-
CLINICAL PHARMACOLOGYTopical corticosteroids share anti-inflammatory, antipruritic and vasoconstrictive actions. The mechanism of anti-inflammatory activity of the topical corticosteroids is unclear. Various ...
-
PharmacokineticsThe extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings ...
-
INDICATIONS AND USAGETopical corticosteroids are indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses.
-
CONTRAINDICATIONSTopical corticosteroids are contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation.
- PRECAUTIONS
-
GeneralSystemic absorption of topical corticosteroids has produced reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, manifestations of Cushing's syndrome, hyperglycemia, and glucosuria in ...
-
Information for PatientsPatients using topical corticosteroids should receive the following information and instructions: 1. This medication is to be used as directed by the physician.. It is for external use ...
-
Laboratory testsThe following tests may be helpful in evaluating the HPA axis suppression: Urinary free cortisol test - ACTH stimulation test
-
Carcinogenesis, Mutagenesis and Impairment of FertilityLong-term animal studies have not been performed to evaluate the carcinogenic potential or the effect on fertility of topical corticosteroids. Studies to determine mutagenicity with ...
-
PregnancyTeratogenic Effects— Pregnancy Category C - Corticosteroids are generally teratogenic in laboratory animals when administered systemically at relatively low dosage levels. The more potent ...
-
Nursing MothersIt is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in breast milk. Systemically administered ...
-
Pediatric UsePediatric patients may demonstrate greater susceptibility to topical corticosteroid-induced HPA axis suppression and Cushing's syndrome than mature patients because of a larger skin surface area ...
-
ADVERSE REACTIONSThe following local adverse reactions are reported infrequently with topical corticosteroids, but may occur more frequently with the use of occlusive dressings. These reactions are listed in an ...
-
OVERDOSAGETopically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects (See - PRECAUTIONS).
-
DOSAGE AND ADMINISTRATIONApply a thin film of Betamethasone Valerate Cream or Ointment to the affected skin areas one to three times a day. Dosage once or twice a day is often effective.
-
HOW SUPPLIEDBetamethasone Valerate Cream USP, 0.1% is supplied as follows: 15 g tubes NDC 0713-0326-15 - 45 g tubes NDC 0713-0326-37 - Betamethasone Valerate Ointment USP, 0.1% is supplied as ...
-
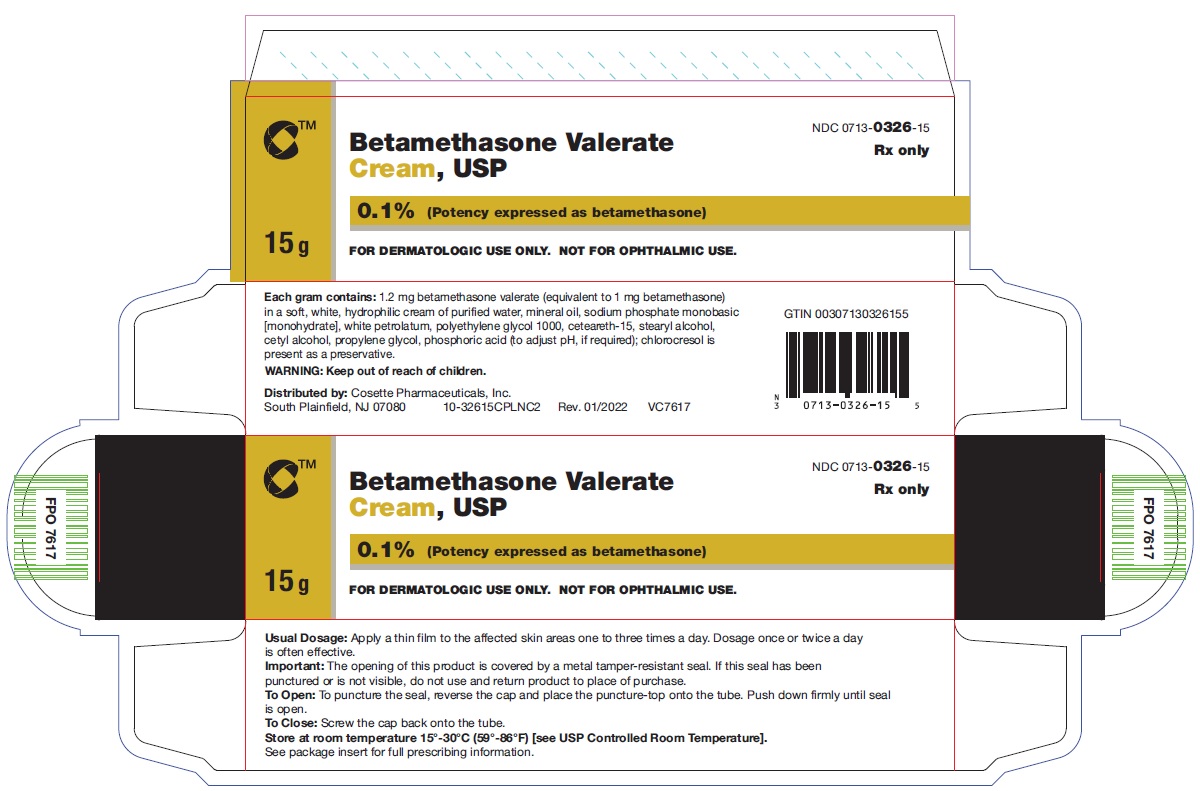
PRINCIPAL DISPLAY PANELNDC 0713-0326-15 - Betamethasone Valerate Cream, USP 0.1% 15 g - Rx only - FOR DERMATOLOGIC USE ONLY. NOT FOR OPHTHALMIC USE. Cosette Pharmaceuticals, Inc. NDC ...
-
INGREDIENTS AND APPEARANCEProduct Information