Label: ALBUTEROL SULFATE INHALATION SOLUTION- albuterol sulfate solution
- NDC Code(s): 67296-1867-7
- Packager: RedPharm Drug
- This is a repackaged label.
- Source NDC Code(s): 76204-010
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
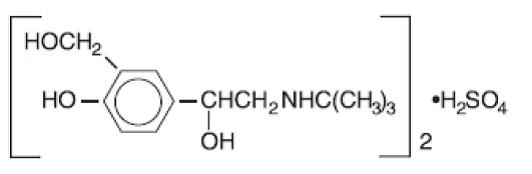
Albuterol sulfate inhalation solution is a sterile, clear, colorless solution of the sulfate salt of racemic albuterol, albuterol sulfate. Albuterol sulfate is a relatively selective beta - 2 ...
-
CLINICAL PHARMACOLOGY
The prime action of beta-adrenergic drugs is to stimulate adenyl cyclase, the enzyme which catalyzes the formation of cyclic-3’,5’-adenosine monophosphate (cyclic AMP) from adenosine triphosphate ...
-
INDICATIONS AND USAGE
Albuterol Sulfate Inhalation Solution is indicated for the relief of bronchospasm in patients 2 to 12 years of age with asthma (reversible obstructive airway disease).
-
CONTRAINDICATIONS
Albuterol Sulfate Inhalation Solution is contraindicated in patients with a history of hypersensitivity to any of its components.
-
WARNINGS
Paradoxical Bronchospasm - As with other inhaled beta-adrenergic agonists, Albuterol Sulfate Inhalation Solution can produce paradoxical bronchospasm, which may be life threatening. If ...
-
PRECAUTIONS
General - Large doses of intravenous albuterol have been reported to aggravate pre-existing diabetes mellitus and ketoacidosis. As with other beta-agonists, inhaled and intravenous albuterol ...
-
ADVERSE REACTIONS
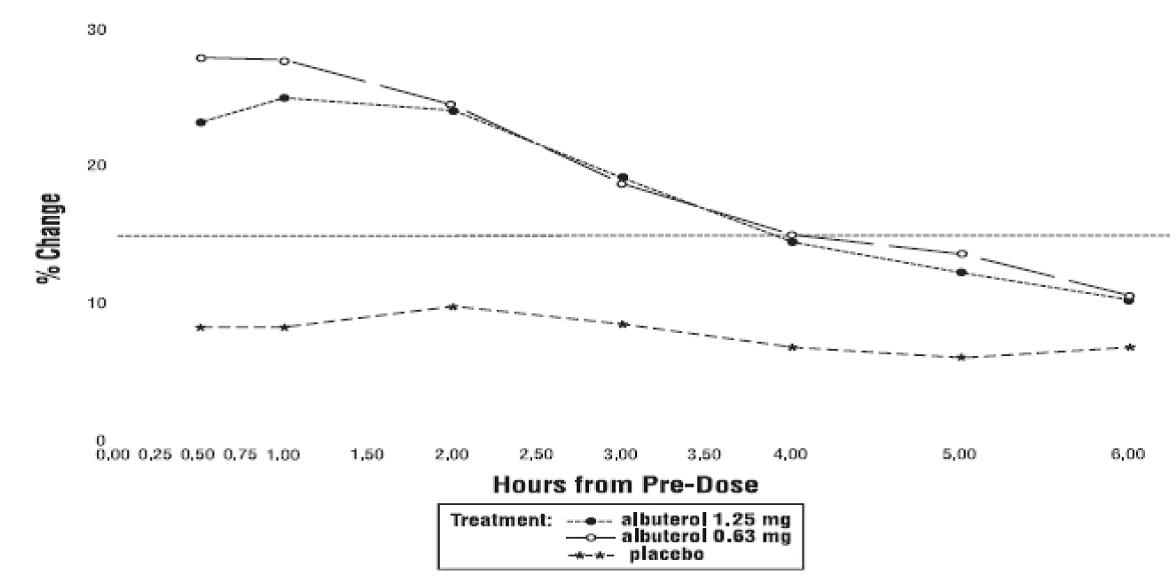
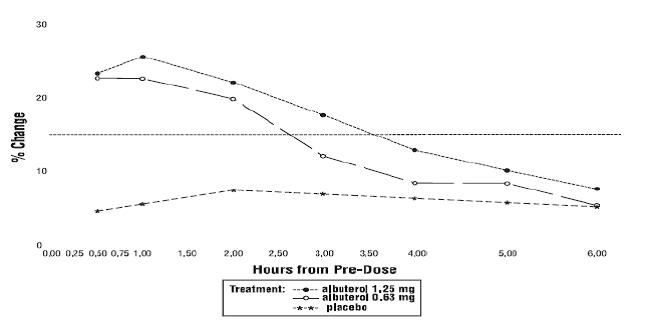
Clinical Trial Experience - Adverse events reported in >1% of patients receiving Albuterol Sulfate Inhalation Solution and more frequently than in patients receiving placebo in a four-week ...
-
OVERDOSAGE
The expected symptoms with overdosage are those of excessive beta-adrenergic stimulation and/or occurrence or exaggeration of symptoms such as seizures, angina, hypertension or hypotension ...
-
DOSAGE AND ADMINISTRATION
The usual starting dosage for patients 2 to 12 years of age is 1.25 mg or 0.63 mg of Albuterol Sulfate Inhalation Solution administered 3 or 4 times daily, as needed, by nebulization. More ...
-
HOW SUPPLIED
Albuterol Sulfate Inhalation Solution is supplied as a 3 mL, clear, colorless, sterile, preservative-free, aqueous solution in two different strengths, 0.63 mg/3 mL and 1.25 mg/3 mL, of albuterol ...
-
STORAGE
Store between 2°C to 25°C (36°F to 77°F). Protect from light and excessive heat. Store unit-dose vials in protective foil pouch at all times. Once removed from the foil pouch, use vial(s) within ...
-
PATIENT INFORMATION
Albuterol Sulfate Inhalation Solution - (al bue' ter ol sul' fate) 0.63 mg*/3 mL and 1.25 mg*/3 mL - (*Equivalent to 0.75 mg of albuterol sulfate or 1.5 mg of albuterol sulfate per 3 ...
-
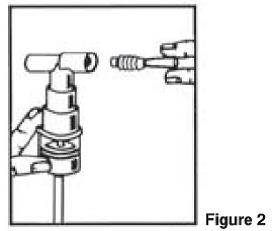
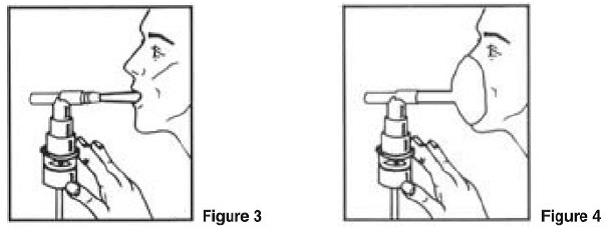
PATIENT'S INSTRUCTIONS FOR USE
Albuterol Sulfate Inhalation Solution - 0.63 mg*/3 mL and 1.25 mg*/3 mL - (*Equivalent to 0.75 mg of albuterol sulfate or 1.5 mg of albuterol sulfate per 3 mL) Read this patient information ...
-
0.63 mg 25 Count Brick Carton
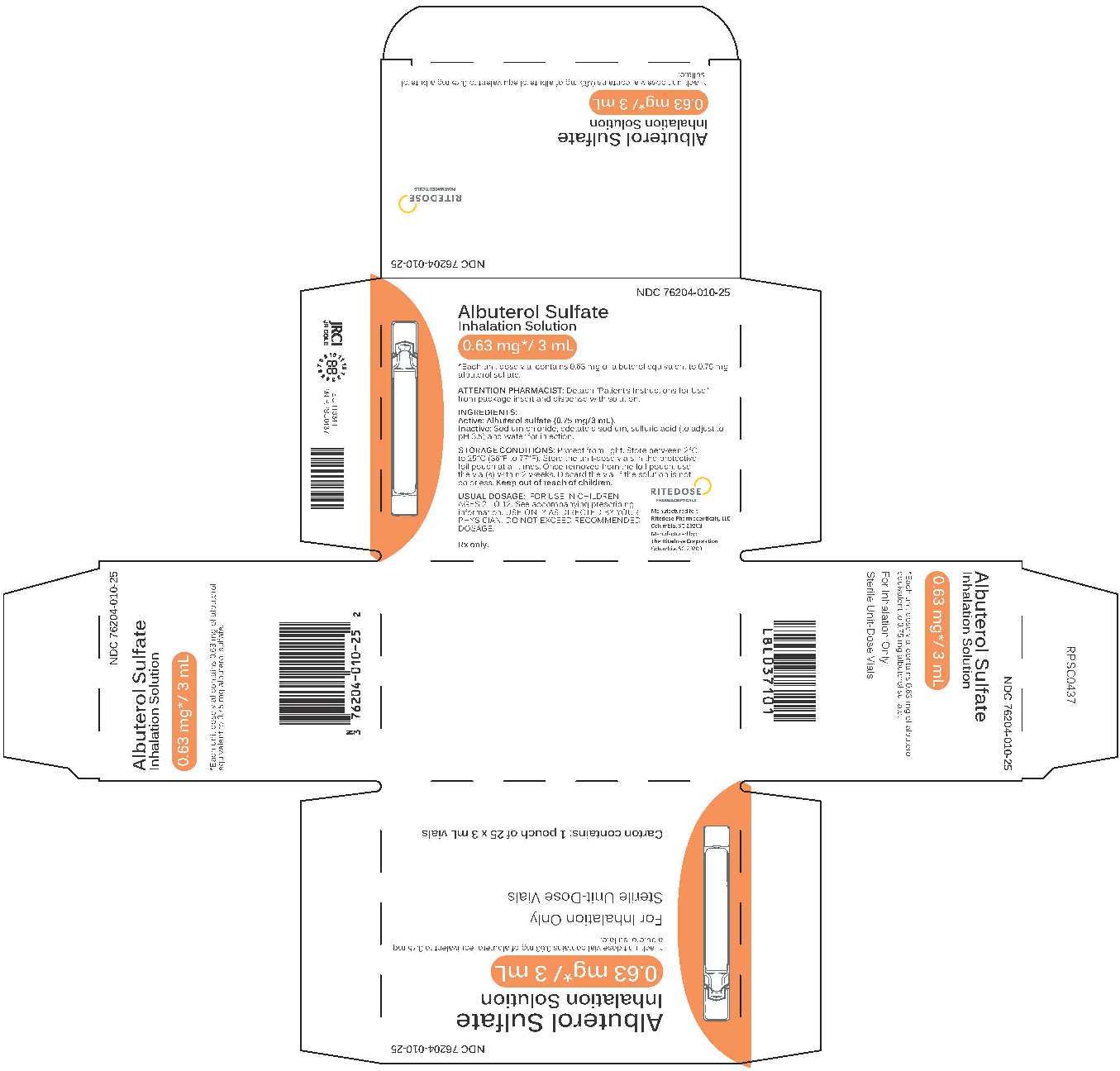
-
0.63 mg 25 Count Cards Carton
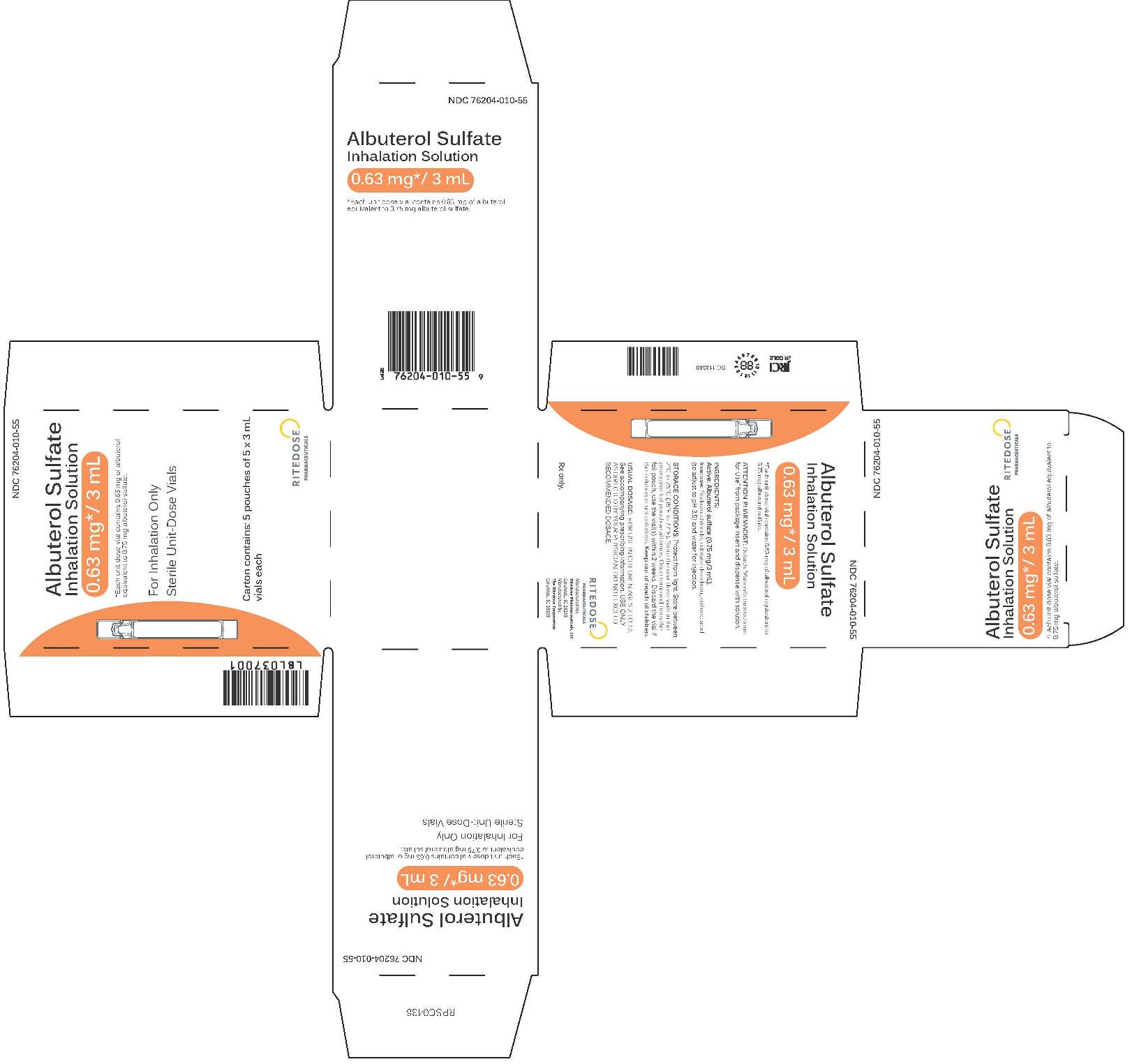
-
0.63 mg 30 Count Singles Carton
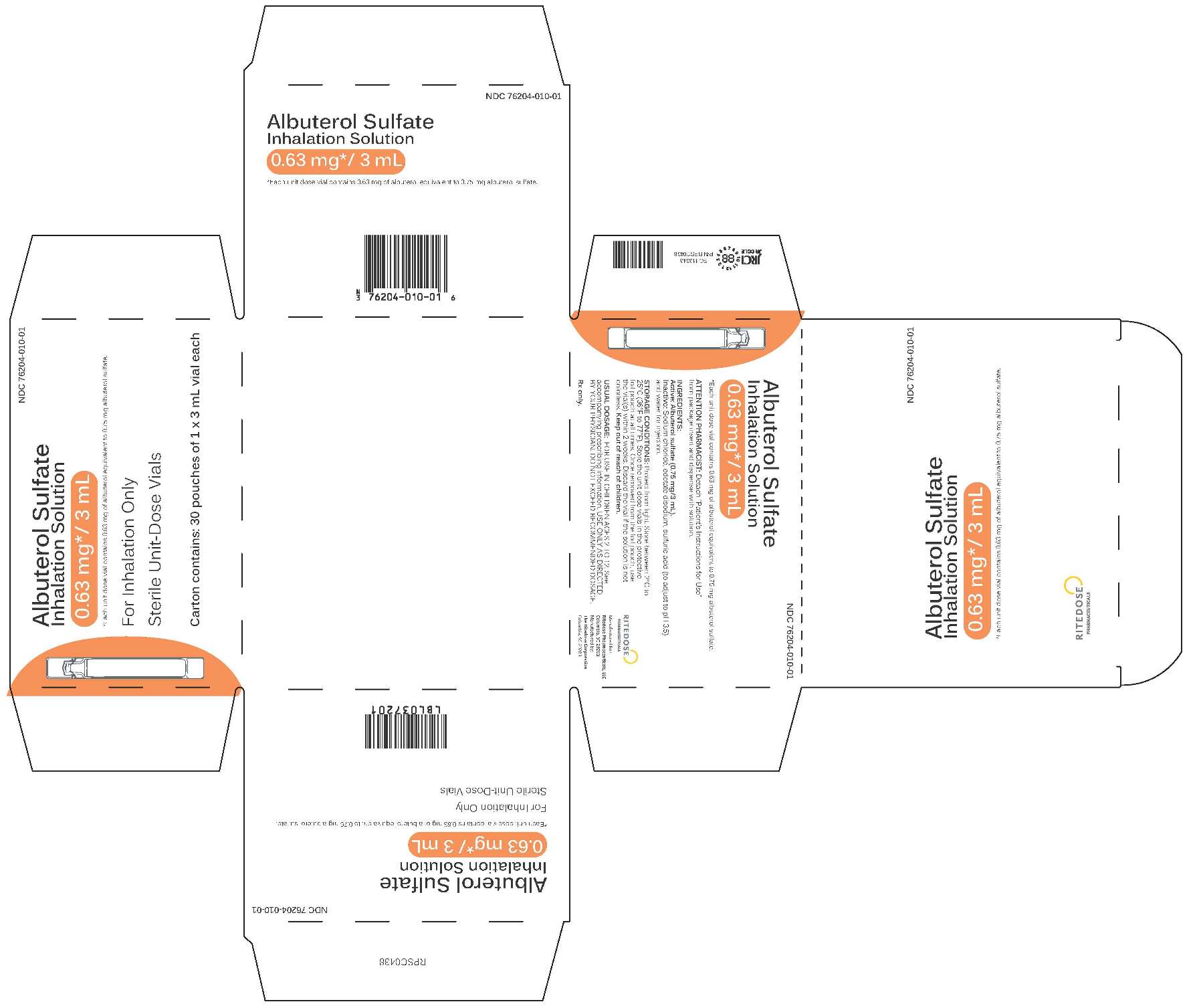
-
1.25 mg 25 count Brick Carton
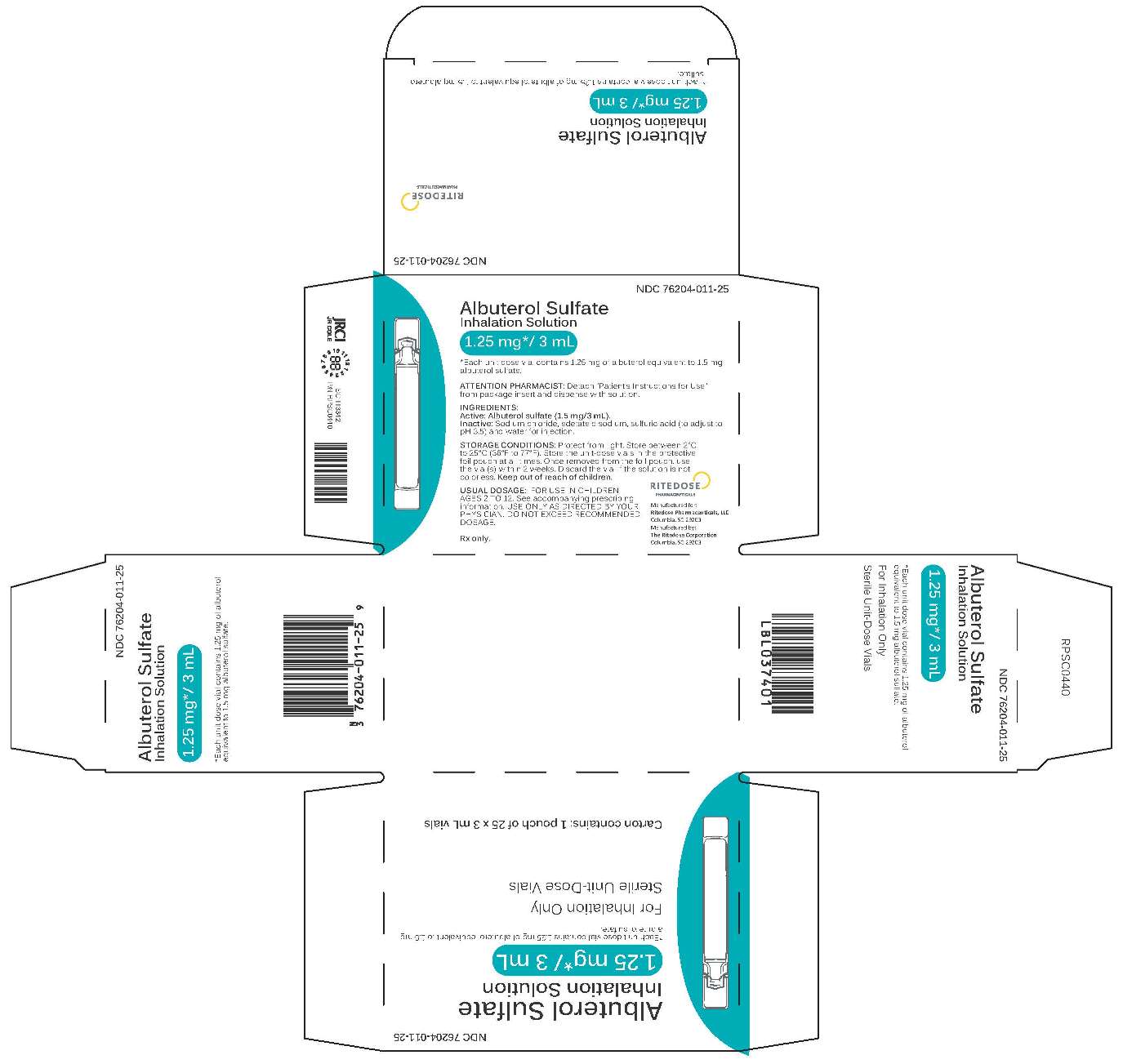
-
1.25 mg 25 Count Cards Carton
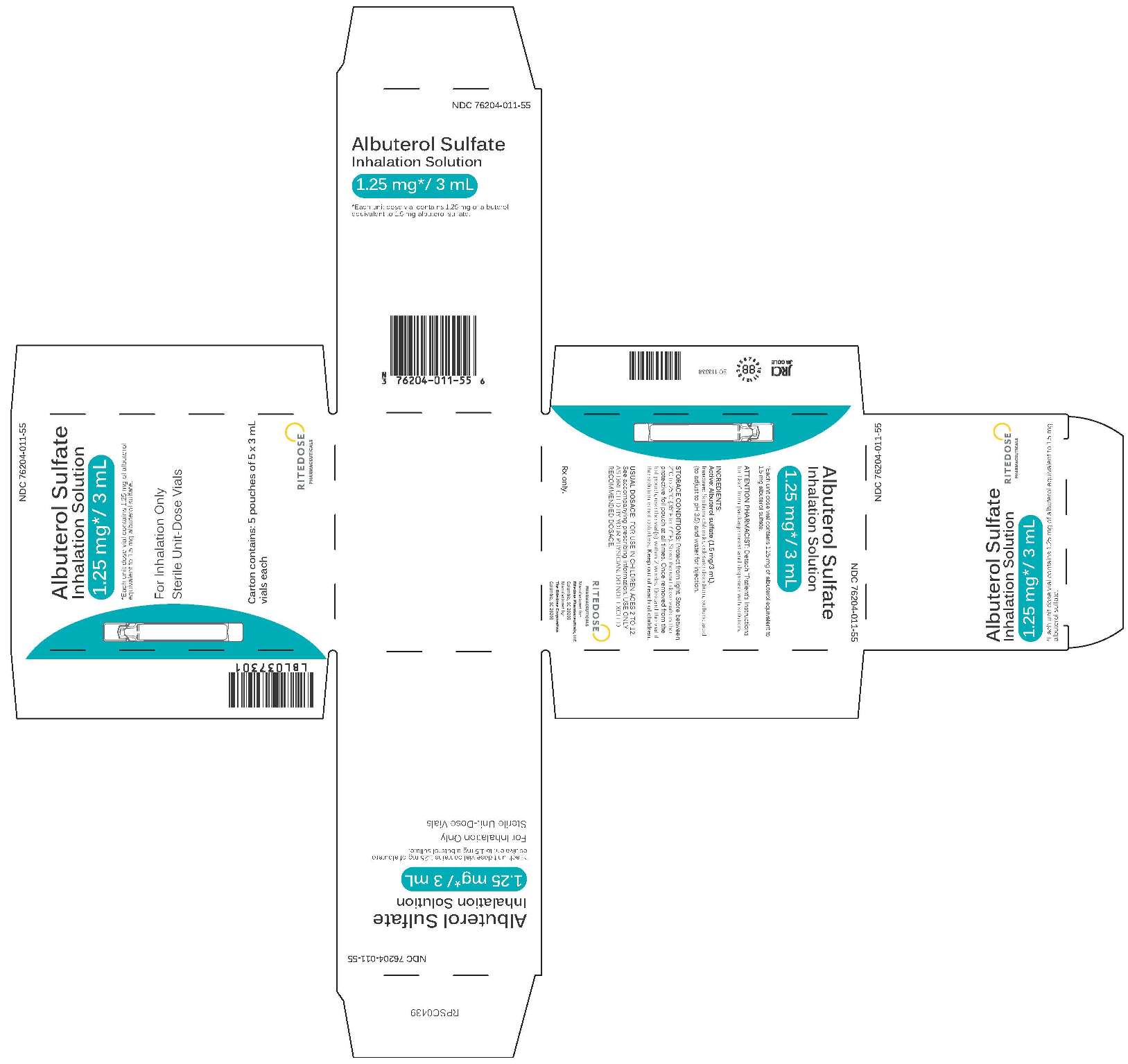
-
1.25 mg 30 Count Singles Carton
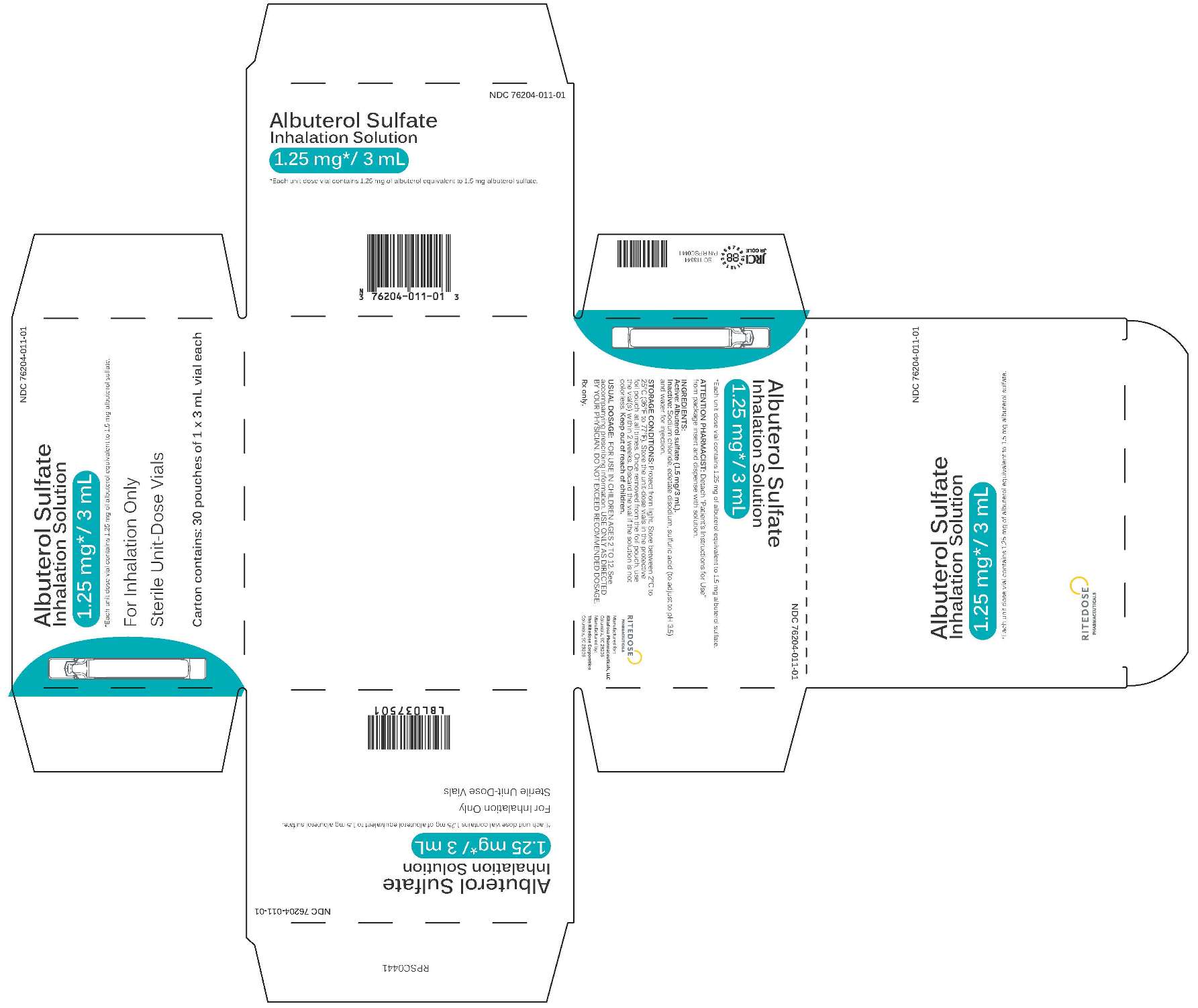
-
INGREDIENTS AND APPEARANCEProduct Information