Label: ADRENALIN- epinephrine injection
- NDC Code(s): 70518-1342-0, 70518-1342-1
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 42023-159
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 22, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ADRENALIN safely and effectively. See full prescribing information for ADRENALIN. ADRENALIN (epinephrine injection) 1 mg/mL ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Anaphylaxis - Emergency treatment of allergic reactions (Type I), including anaphylaxis, which may result from insect stings or bites, foods, drugs, sera, diagnostic testing substances ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Considerations - Inspect visually for particulate matter and discoloration prior to administration; solution should be clear and colorless. Do not use if the solution is colored ...
-
3 DOSAGE FORMS AND STRENGTHSAdrenalin injection: clear, colorless solution supplied as 1 mg/1 mL in a single dose clear glass vial and as 30 mg/30 mL (1 mg/mL) in a multiple dose amber glass vial.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Incorrect Locations of Injection for Anaphylaxis - Injection into the anterolateral aspect of the thigh (vastus lateralis muscle) is the most appropriate location for administration because ...
-
6 ADVERSE REACTIONSCommon adverse reactions to systemically administered epinephrine include anxiety, apprehensiveness, restlessness, tremor, weakness, dizziness, sweating, palpitations, pallor, nausea and vomiting ...
-
7 DRUG INTERACTIONS7.1 Drugs Antagonizing Pressor Effects of Epinephrine - α-blockers, such as phentolamine - Vasodilators, such as nitrates - Diuretics - Antihypertensives - Ergot alkaloids - Phenothiazine ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Prolonged experience with epinephrine use in pregnant women over several decades, based on published literature, do not identify a drug associated risk of major ...
-
10 OVERDOSAGEOverdosage of epinephrine may produce extremely elevated arterial pressure, which may result in cerebrovascular hemorrhage, particularly in elderly patients. Overdosage may also result in ...
-
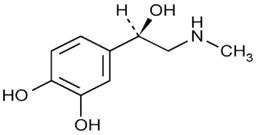
11 DESCRIPTIONAdrenalin (epinephrine injection, USP) is a clear, colorless, sterile solution containing 1 mg/mL epinephrine, packaged as 1 mL of solution in a single dose clear glass vial or 30 mL of solution ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Epinephrine acts on both alpha and beta-adrenergic receptors. The mechanism of the rise in blood pressure is 3-fold: a direct myocardial stimulation that increases the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies to evaluate the carcinogenic potential of epinephrine have not been conducted. Epinephrine and other catecholamines ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAdrenalin 1 mg/mL Single Dose Vials: Each carton contains 25 single dose vials containing 1 mg/mL Adrenalin (epinephrine injection, USP) solution in a 3 mL clear glass vial. NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients or their caregivers about common adverse reactions associated with the use of epinephrine including an increase in heart rate, the sensation of a more forceful heartbeat ...
-
PRINCIPAL DISPLAY PANELDRUG: Adrenalin - GENERIC: epinephrine - DOSAGE: INJECTION - ADMINSTRATION: INTRAMUSCULAR - NDC: 70518-1342-0 - NDC: 70518-1342-1 - PACKAGING: 1 mL in 1 VIAL - OUTER PACKAGING: 25 in 1 CARTON - ACTIVE ...
-
INGREDIENTS AND APPEARANCEProduct Information