Label: ALBUTEROL SULFATE solution
- NDC Code(s): 81894-105-25, 81894-105-30, 81894-106-25, 81894-106-30
- Packager: Luoxin Aurovitas Pharma (Chengdu) Co., Ltd.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
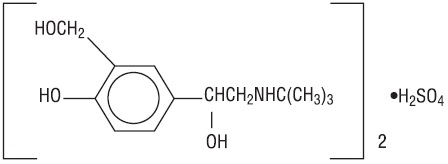
DESCRIPTIONAlbuterol sulfate inhalation solution is a sterile, clear, colorless solution of the sulfate salt of racemic albuterol, albuterol sulfate. Albuterol sulfate is a relatively selective beta ...
-
CLINICAL PHARMACOLOGYThe prime action of beta-adrenergic drugs is to stimulate adenyl cyclase, the enzyme which catalyzes the formation of cyclic-3',5'-adenosine monophosphate (cyclic AMP) from adenosine triphosphate ...
-
INDICATIONS AND USAGEAlbuterol sulfate inhalation solution is indicated for the relief of bronchospasm in patients 2 to 12 years of age with asthma (reversible obstructive airway disease).
-
CONTRAINDICATIONSAlbuterol sulfate inhalation solution is contraindicated in patients with a history of hypersensitivity to any of its components.
-
WARNINGSParadoxical Bronchospasm:As with other inhaled beta-adrenergic agonists, albuterol sulfate inhalation solution can produce paradoxical bronchospasm, which may be life threatening. If paradoxical ...
-
PRECAUTIONSGeneral - Large doses of intravenous albuterol have been reported to aggravate pre-existing diabetes mellitus and ketoacidosis. As with other beta-agonists, inhaled and intravenous albuterol may ...
-
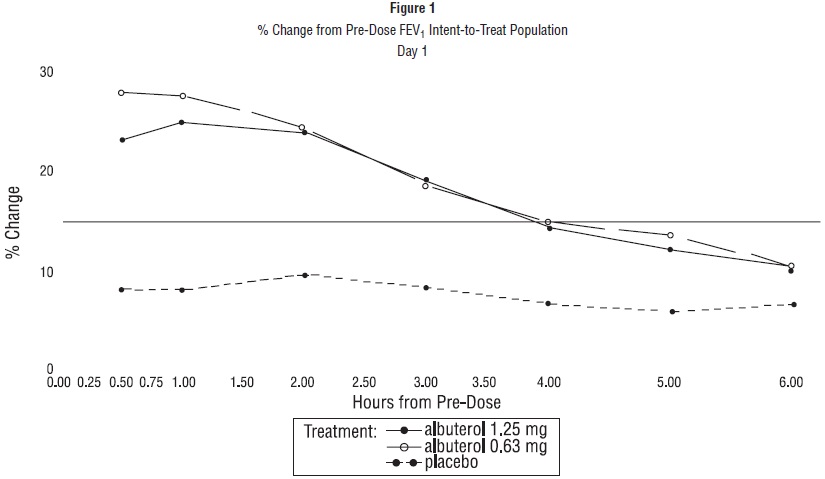
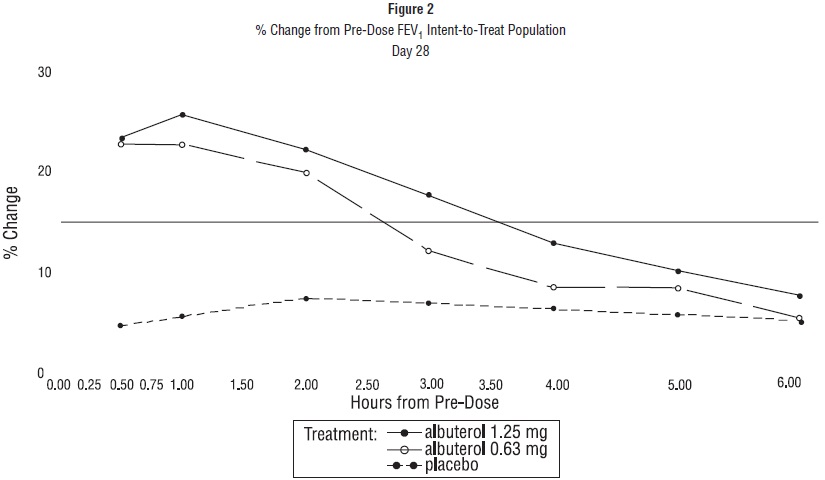
ADVERSE REACTIONSClinical Trial Experience: Adverse events reported in >1% of patients receiving albuterol sulfate inhalation solution and more frequently than in patients receiving placebo in a four-week double ...
-
OVERDOSAGEThe expected symptoms with overdosage are those of excessive beta-adrenergic stimulation and/or occurrence or exaggeration of symptoms such as seizures, angina, hypertension or hypotension ...
-
DOSAGE AND ADMINISTRATIONThe usual starting dosage for patients 2 to 12 years of age is 1.25 mg or 0.63 mg of albuterol sulfate inhalation solution administered 3 or 4 times daily, as needed, by nebulization. More ...
-
HOW SUPPLIEDAlbuterol Sulfate Inhalation Solution is supplied as a 3 mL, clear, colorless, sterile, preservative-free, aqueous solution in two different strengths, 0.63 mg/3 mL and 1.25 mg/3 mL, of albuterol ...
-
STORAGEStore between 2°C to 25°C (36°F to 77°F). Protect from light and excessive heat. Store unit-dose vials in protective foil pouch at all times. Once removed from the foil pouch, use ...
-
Patient InformationAlbuterol Sulfate Inhalation Solution - (al bue' ter ol sul' fate) 0.63 mg*/3 mL and 1.25 mg*/3 mL - (*Equivalent to 0.75 mg of albuterol sulfate or 1.5 mg of albuterol sulfate per 3 ...
-
Albuterol Sulfate Inhalation Solution
0.63 mg*/3 mL and 1.25 mg*/3 mL
(*Equivalent to 0.75 mg of albuterol sulfate or 1.5 mg of albuterol sulfate per 3 mL)
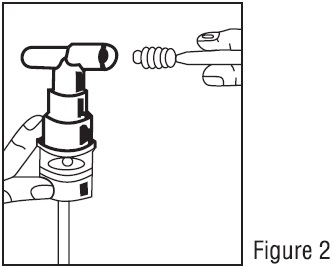
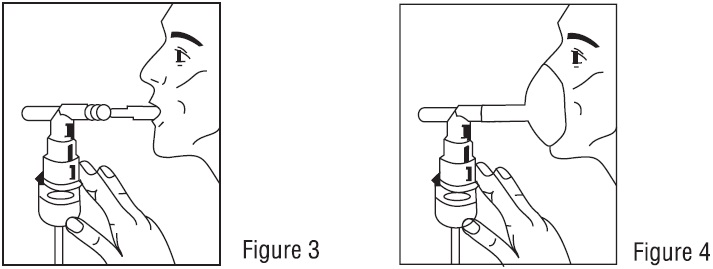
PATIENT'S INSTRUCTIONS FOR USE
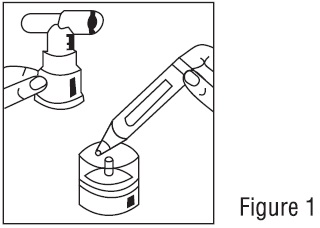
Read this patient information completely every time your prescription is filled as information may have changed. Keep these instructions with your medication, as you may want to read them ...
-
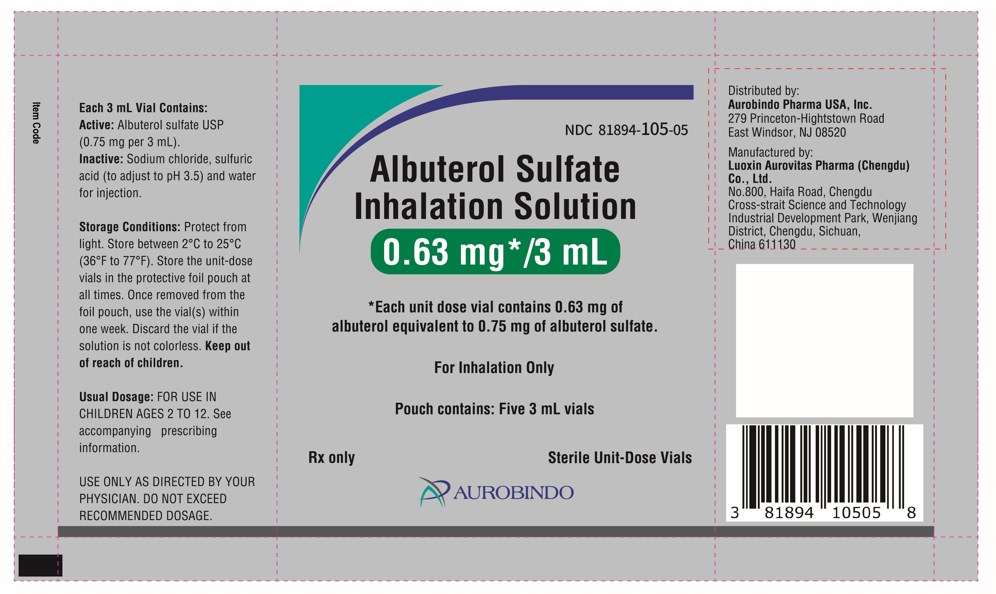
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 0.63 mg per 3 mL - Pouch Label (5 Vials)NDC 81894-105-05 - Albuterol Sulfate - Inhalation Solution - 0.63 mg*/3 mL - * Each unit dose vial contains 0.63 mg of - albuterolequivalent to 0.75 mg of albuterol sulfate. For ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 0.63 mg per 3 mL – Container-Carton (25 Vials)NDC 81894-105-25 - Albuterol Sulfate - Inhalation Solution - 0.63 mg*/3 mL - * Each unit dose vial contains 0.63 mg of albuterol - equivalent to 0.75 mg of albuterol ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 0.63 mg per 3 mL – Container-Carton (30 Vials)NDC 81894-105-30 - Albuterol Sulfate - Inhalation Solution - 0.63 mg*/3 mL - * Each unit dose vial contains 0.63 mg of albuterol - equivalent to 0.75 mg of albuterol ...
-
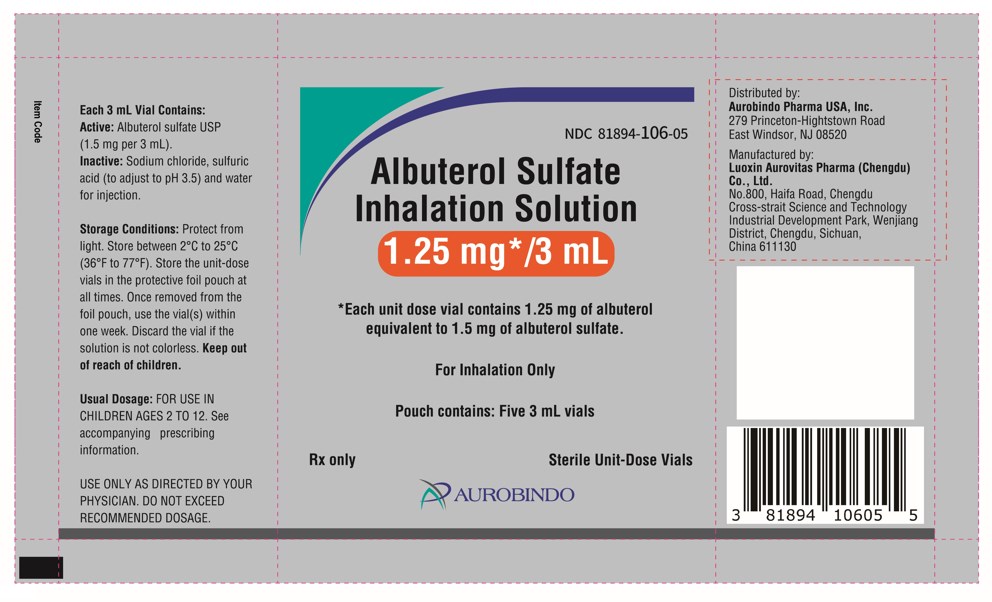
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL – 1.25 mg per 3 mL - Pouch Label (5 Vials)NDC 81894-106-05 - Albuterol Sulfate - Inhalation Solution - 1.25 mg*/3 mL - * Each unit dose vial contains 1.25 mg of albuterol - equivalent to 1.5 mg of albuterol sulfate. For ...
-
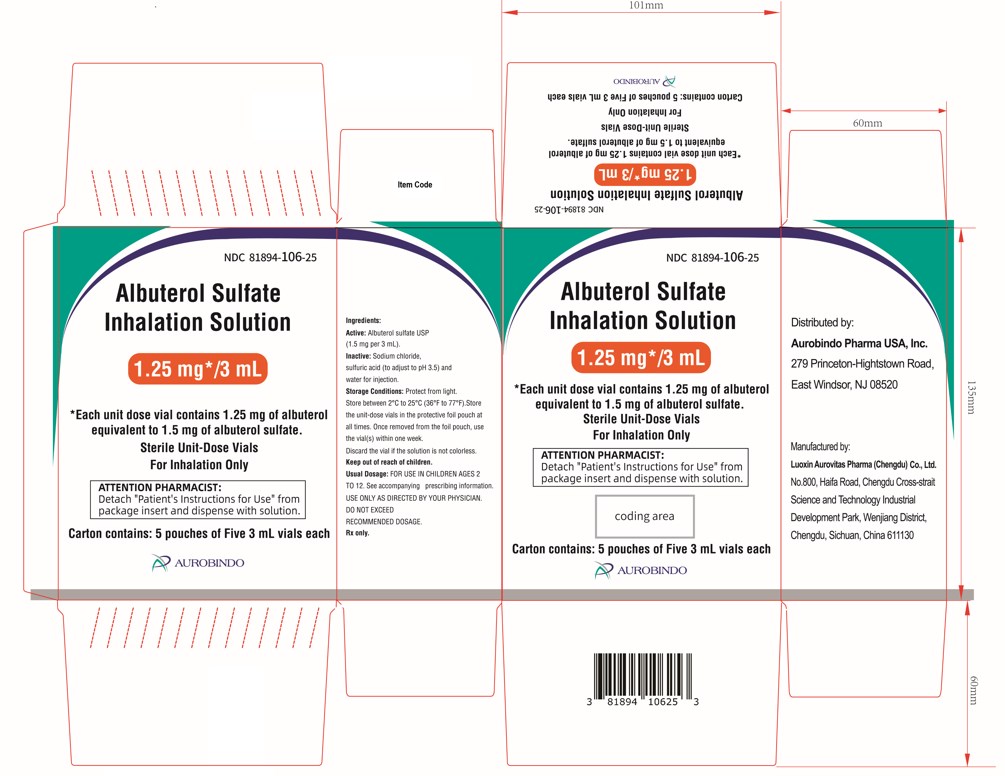
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1.25 mg per 3 mL – Container-Carton (25 Vials)NDC 81894-106-25 - Albuterol Sulfate - Inhalation Solution - 1.25 mg*/3 mL - * Each unit dose vial contains 1.25 mg of albuterol - equivalent to 1.5 mg of albuterol sulfate. Sterile ...
-
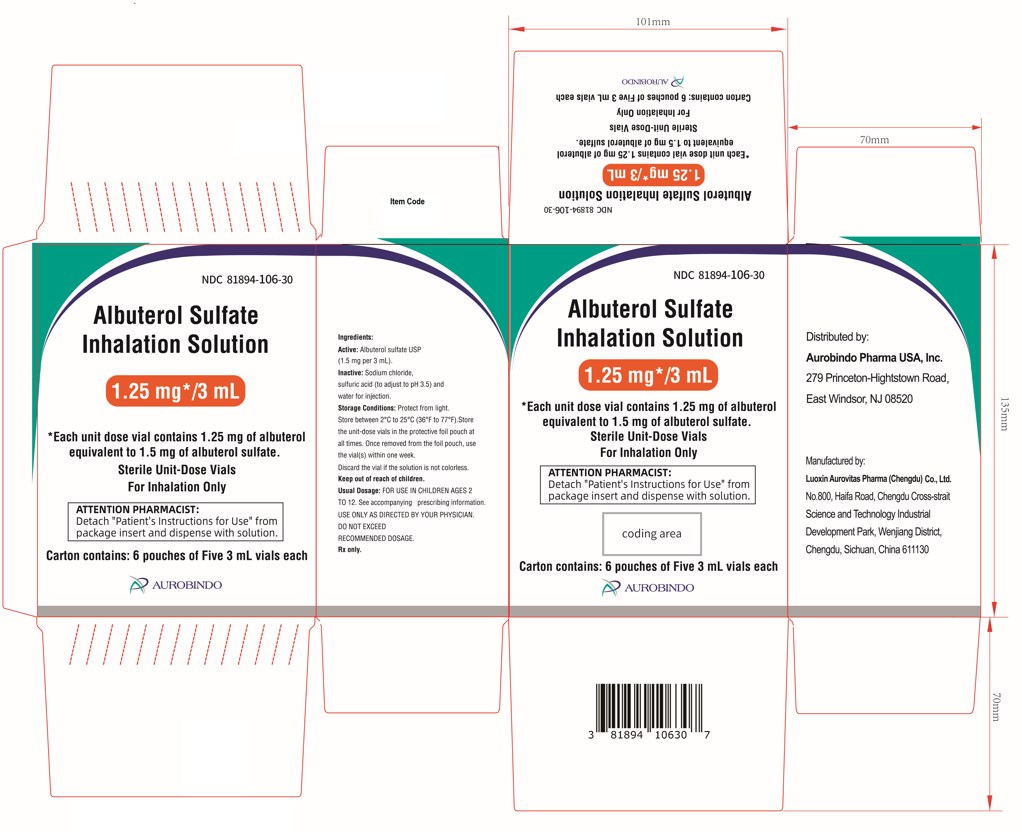
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1.25 mg per 3 mL – Container-Carton (30 Vials)NDC 81894-106-30 - Albuterol Sulfate - Inhalation Solution - 0.63 mg*/3 mL - * Each unit dose vial contains 0.63 mg of albuterol - equivalent to 0.75 mg of albuterol ...
-
INGREDIENTS AND APPEARANCEProduct Information