Label: AVANAFIL- avanafil tablet
-
NDC Code(s):
31722-440-01,
31722-440-30,
31722-441-01,
31722-441-30, view more31722-442-01, 31722-442-30
- Packager: Camber Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AVANAFIL TABLETS safely and effectively. See full prescribing information for AVANAFIL TABLETS. AVANAFIL tablets, for oral use ...These highlights do not include all the information needed to use AVANAFIL TABLETS safely and effectively. See full prescribing information for AVANAFIL TABLETS.
AVANAFIL tablets, for oral use
Initial U.S. Approval: 2012INDICATIONS AND USAGE
Avanafil tablet is a phosphodiesterase 5 (PDE5) inhibitor indicated for the treatment of erectile dysfunction ( 1)
DOSAGE AND ADMINISTRATION
• The starting dose is 100 mg taken as early as approximately 15 minutes before sexual activity, on an as needed basis ( 2.1)
• Take avanafil tablet no more than once a day ( 2.1).
• Based on efficacy and/or tolerability, the dose may be increased to 200 mg taken as early as approximately 15 minutes before sexual activity or decreased to 50 mg taken approximately 30 minutes before sexual activity. Use the lowest dose that provides benefit ( 2.1).
• Avanafil tablet may be taken with or without food ( 2.2)
• Do not use avanafil tablet with strong CYP3A4 inhibitors ( 2.3)
• If taking a moderate CYP3A4 inhibitor, the dose should be no more than 50 mg in a 24-hour period ( 2.3).
• In patients on stable alpha-blocker therapy, the recommended starting dose of avanafil tablet is 50 mg ( 2.3).DOSAGE FORMS AND STRENGTHS
Tablets: 50 mg, 100 mg, 200 mg ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
• Patients should not use avanafil if sexual activity is inadvisable due to cardiovascular status or any other reason ( 5.1)
• Use of avanafil with alpha-blockers, other antihypertensives, or substantial amounts of alcohol (greater than 3 units) may lead to hypotension ( 2.3, 5.6, 5.7)
• Patients should seek emergency treatment if an erection lasts greater than 4 hours ( 5.3)
• Patients should stop avanafil and seek medical care if a sudden loss of vision occurs in one or both eyes, which could be a sign of Non Arteritic Ischemic Optic Neuropathy (NAION). Avanafil should be used with caution, and only when the anticipated benefits outweigh the risks, in patients with a history of NAION. Patients with a “crowded” optic disc may also be at an increased risk of NAION ( 5.4, 6.2)
• Patients should stop taking avanafil and seek prompt medical attention in the event of sudden decrease or loss of hearing ( 5.5)ADVERSE REACTIONS
Most common adverse reactions (greater than or equal to 2%) include headache, flushing, nasal congestion, nasopharyngitis, and back pain ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Hetero Labs Limited at 1-866-495-1995 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2024
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Erectile Dysfunction
2.2 Use with Food
2.3 Concomitant Medications
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Nitrates
4.2 Hypersensitivity Reactions
4.3 Concomitant Guanylate Cyclase (GC) Stimulators
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Risk
5.2 Concomitant Use of CYP3A4 Inhibitors
5.3 Prolonged Erection
5.4 Effects on Eye
5.5 Sudden Hearing Loss
5.6 Alpha-Blockers and Other Antihypertensives
5.7 Alcohol
5.8 Combination with Other PDE5 Inhibitors or Erectile Dysfunction Therapies
5.9 Effects on Bleeding
5.10 Counseling Patients about Sexually Transmitted Diseases
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Potential for Pharmacodynamic Interactions with Avanafil
7.2 Potential for Other Drugs to Affect Avanafil
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Nitrates
17.2 Cardiovascular Considerations
17.3 Concomitant Use with Drugs Which Lower Blood Pressure
17.4 Potential for Drug Interactions
17.5 Priapism
17.6 Vision
17.7 Sudden Hearing Loss
17.8 Alcohol
17.9 Sexually Transmitted Disease
17.10 Recommended Administration
17.11 Guanylate Cyclase (GC) Stimulators
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGEAvanafil tablets are a phosphodiesterase 5 (PDE5) inhibitor indicated for the treatment of erectile dysfunction in adult males.
Avanafil tablets are a phosphodiesterase 5 (PDE5) inhibitor indicated for the treatment of erectile dysfunction in adult males.
Close -
2 DOSAGE AND ADMINISTRATION2.1 Erectile Dysfunction - The recommended starting dose is 100 mg. Avanafil tablet should be taken orally as needed as early as approximately 15 minutes before sexual activity. Based ...
2.1 Erectile Dysfunction
The recommended starting dose is 100 mg. Avanafil tablet should be taken orally as needed as early as approximately 15 minutes before sexual activity.
Based on individual efficacy and tolerability, the dose may be increased to 200 mg taken as early as approximately 15 minutes before sexual activity, or decreased to 50 mg taken approximately 30 minutes before sexual activity. The lowest dose that provides benefit should be used.
The maximum recommended dosing frequency is once per day. Sexual stimulation is required for a response to treatment.Close2.3 Concomitant Medications
Nitrates
Concomitant use of nitrates in any form is contraindicated [see Contraindications ( 4.1)].
Alpha-Blockers
If avanafil tablet is co-administered with an alpha-blocker, patients should be stable on alpha-blocker therapy prior to initiating treatment with avanafil tablet, and avanafil tablet should be initiated at the 50 mg dose [see Warnings and Precautions ( 5.6), Drug Interactions ( 7.1) and Clinical Pharmacology ( 12.2)].
CYP3A4 Inhibitors
• For patients taking concomitant strong CYP3A4 inhibitors (including ketoconazole, ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir and telithromycin), do not use avanafil tablet [see Warnings and Precautions ( 5.2) and Drug Interactions ( 7.2)].
• For patients taking concomitant moderate CYP3A4 inhibitors (including erythromycin, amprenavir, aprepitant, diltiazem, fluconazole, fosamprenavir, and verapamil), the maximum recommended dose of avanafil tablet is 50 mg, not to exceed once every 24 hours [see Warnings and Precautions ( 5.2) and Drug Interactions ( 7.2)]. -
3 DOSAGE FORMS AND STRENGTHSAvanafil tablets, 50 mg are white to off white, oval shape, biconvex tablets debossed with ‘A41’ on one side and ‘H’ on the other side. Avanafil tablets, 100 mg are white to off white ...
Avanafil tablets, 50 mg are white to off white, oval shape, biconvex tablets debossed with ‘A41’ on one side and ‘H’ on the other side.
Close
Avanafil tablets, 100 mg are white to off white, oval shape, biconvex tablets debossed with ‘A42’ on one side and ‘H’ on the other side.
Avanafil tablets, 200 mg are white to off white, oval shape, biconvex tablets debossed with ‘A43’ on one side and ‘H’ on the other side. -
4 CONTRAINDICATIONS4.1 Nitrates - Administration of avanafil tablets with any form of organic nitrates, either regularly and/or intermittently, is contraindicated. Consistent with its known effects on the nitric ...
4.1 Nitrates
Administration of avanafil tablets with any form of organic nitrates, either regularly and/or intermittently, is contraindicated. Consistent with its known effects on the nitric oxide/cyclic guanosine monophosphate (cGMP) pathway, avanafil has been shown to potentiate the hypotensive effects of nitrates.
In a patient who has taken avanafil tablets, where nitrate administration is deemed medically necessary in a life-threatening situation, at least 12 hours should elapse after the last dose of avanafil tablets before nitrate administration is considered. In such circumstances, nitrates should only be administered under close medical supervision with appropriate hemodynamic monitoring [see Contraindications ( 4.1), Dosage and Administration ( 2.3), and Clinical Pharmacology ( 12.2)].4.2 Hypersensitivity Reactions
Avanafil is contraindicated in patients with a known hypersensitivity to any component of the tablet. Hypersensitivity reactions have been reported, including pruritis and eyelid swelling.
Close4.3 Concomitant Guanylate Cyclase (GC) Stimulators
Do not use avanafil tablets in patients who are using a GC stimulator, such as riociguat or vericiguat. PDE5 inhibitors, including avanafil tablets may potentiate the hypotensive effects of GC stimulators.
-
5 WARNINGS AND PRECAUTIONSEvaluation of erectile dysfunction (ED) should include an appropriate medical assessment to identify potential underlying causes, as well as treatment options. Before prescribing ...
Evaluation of erectile dysfunction (ED) should include an appropriate medical assessment to identify potential underlying causes, as well as treatment options.
Before prescribing avanafil, it is important to note the following:5.1 Cardiovascular Risk
There is a potential for cardiac risk during sexual activity in patients with pre-existing cardiovascular disease. Therefore, treatments for ED, including avanafil, should not be used in men for whom sexual activity is inadvisable because of their underlying cardiovascular status.
Patients with left ventricular outflow obstruction (e.g., aortic stenosis, idiopathic hypertrophic subaortic stenosis) and those with severely impaired autonomic control of blood pressure can be particularly sensitive to the actions of vasodilators, including avanafil.
The following groups of patients were not included in clinical safety and efficacy trials for avanafil, and therefore until further information is available, avanafil is not recommended for the following groups:
• Patients who have suffered a myocardial infarction, stroke, life-threatening arrhythmia, or coronary revascularization within the last 6 months;
• Patients with resting hypotension (blood pressure less than 90/50 mmHg) or hypertension (blood pressure greater than 170/100 mmHg);
• Patients with unstable angina, angina with sexual intercourse, or New York Heart Association Class 2 or greater congestive heart failure.
As with other PDE5 inhibitors avanafil has systemic vasodilatory properties and may augment the blood pressure-lowering effect of other anti-hypertensive medications. Avanafil 200 mg resulted in transient decreases in sitting blood pressure in healthy volunteers of 8.0 mmHg systolic and 3.3 mmHg diastolic [see Clinical Pharmacology ( 12.2)] , with the maximum decrease observed at 1 hour after dosing. While this normally would be expected to be of little consequence in most patients, prior to prescribing avanafil, physicians should carefully consider whether patients with underlying cardiovascular disease could be affected adversely by such vasodilatory effects, especially in combination with sexual activity.5.2 Concomitant Use of CYP3A4 Inhibitors
Avanafil metabolism is principally mediated by the CYP450 isoform 3A4 (CYP3A4). Inhibitors of CYP3A4 may reduce avanafil clearance and increase plasma concentrations of avanafil.
For patients taking concomitant strong CYP3A4 inhibitors (including ketoconazole, ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir and telithromycin), do not use avanafil [see Drug Interactions ( 7.2)].
For patients taking concomitant moderate CYP3A4 inhibitors (including erythromycin, amprenavir, aprepitant, diltiazem, fluconazole, fosamprenavir, and verapamil), the maximum recommended dose of avanafil is 50 mg, not to exceed once every 24 hours [see Drug Interactions ( 7.2)].5.3 Prolonged Erection
Prolonged erection greater than 4 hours and priapism (painful erections greater than 6 hours in duration) have been reported with other PDE5 inhibitors. In the event of an erection that persists longer than 4 hours, the patient should seek immediate medical assistance. If not treated immediately, penile tissue damage and permanent loss of potency could result.
Avanafil should be used with caution in patients with anatomical deformation of the penis (such as angulation, cavernosal fibrosis, or Peyronie’s disease), or in patients who have conditions which may predispose them to priapism (such as sickle cell anemia, multiple myeloma, or leukemia).5.4 Effects on Eye
Physicians should advise patients to stop use of all PDE5 inhibitors, including avanafil and seek medical attention in the event of a sudden loss of vision in one or both eyes. Such an event may be a sign of non-arteritic anterior ischemic optic neuropathy (NAION), a rare condition and a cause of decreased vision including permanent loss of vision that has been reported rarely postmarketing in temporal association with the use of all PDE5 inhibitors. Based on published literature, the annual incidence of NAION is 2.5 to 11.8 cases per 100,000 in males aged ≥ 50.
An observational case-crossover study evaluated the risk of NAION when PDE5 inhibitor use, as a class, occurred immediately before NAION onset (within 5 half-lives), compared to PDE5 inhibitor use in a prior time period. The results suggest an approximate 2-fold increase in the risk of NAION, with a risk estimate of 2.15 (95% CI 1.06, 4.34). A similar study reported a consistent result, with a risk estimate of 2.27 (95% CI 0.99, 5.20). Other risk factors for NAION, such as the presence of “crowded” optic disc, may have contributed to the occurrence of NAION in these studies.
Neither the rare postmarketing reports, nor the association of PDE5 inhibitor use and NAION in the observational studies, substantiate a causal relationship between PDE5 inhibitor use and NAION [see Adverse Reactions ( 6.2)].
Physicians should consider whether their patients with underlying NAION risk factors could be adversely affected by use of PDE5 inhibitors. Individuals who have already experienced NAION are at increased risk of NAION recurrence. Therefore, PDE5 inhibitors, including avanafil should be used with caution in these patients and only when the anticipated benefits outweigh the risks. Individuals with “crowded” optic disc are also considered at greater risk for NAION compared to the general population; however, evidence is insufficient to support screening of prospective users of PDE5 inhibitors, including avanafil, for this uncommon condition.
Patients with known hereditary degenerative retinal disorders, including retinitis pigmentosa, were not included in the clinical trials for avanafil. and therefore, until further information is available, avanafil is not recommended for use in these patients.5.5 Sudden Hearing Loss
Use of PDE5 inhibitors has been associated with sudden decrease or loss of hearing, which may be accompanied by tinnitus or dizziness. It is not possible to determine whether these events are related directly to the use of PDE5 inhibitors or to other factors [see Adverse Reactions ( 6)] . Patients experiencing these symptoms should be advised to stop taking avanafil and seek prompt medical attention.
5.6 Alpha-Blockers and Other Antihypertensives
Physicians should discuss with patients the potential for avanafil to augment the blood pressure-lowering effect of alpha-blockers and other antihypertensive medications [see Drug Interactions ( 7.1) and Clinical Pharmacology ( 12.2)].
Caution is advised when PDE5 inhibitors are co-administered with alpha-blockers. Phosphodiesterase type 5 inhibitors, including avanafil, and alpha-adrenergic blocking agents are both vasodilators with blood pressure-lowering effects. When vasodilators are used in combination, an additive effect on blood pressure may be anticipated. In some patients, concomitant use of these two drug classes can lower blood pressure significantly leading to symptomatic hypotension (e.g., dizziness, lightheadedness, fainting).
Consideration should be given to the following:
• Patients should be stable on alpha-blocker therapy prior to initiating treatment with a PDE5 inhibitor. Patients who demonstrate hemodynamic instability on alpha-blocker therapy alone are at increased risk of symptomatic hypotension with concomitant use of PDE5 inhibitors.
• In those patients who are stable on alpha-blocker therapy, PDE5 inhibitors should be initiated at the lowest dose (Avanafil 50 mg).
• In those patients already taking an optimized dose of a PDE5 inhibitor, alpha-blocker therapy should be initiated at the lowest dose. Stepwise increase in alpha-blocker dose may be associated with further lowering of blood pressure when taking a PDE5 inhibitor.
Safety of combined use of PDE5 inhibitors and alpha-blockers may be affected by other variables, including intravascular volume depletion and other anti-hypertensive drugs [see Dosage and Administration ( 2) and Drug Interactions ( 7.1)].5.7 Alcohol
Patients should be made aware that both alcohol and PDE5 inhibitors including avanafil act as vasodilators. When vasodilators are taken in combination, blood-pressure-lowering effects of each individual compound may be increased. Therefore, physicians should inform patients that substantial consumption of alcohol (e.g., greater than 3 units) in combination with avanafil may increase the potential for orthostatic signs and symptoms, including increase in heart rate, decrease in standing blood pressure, dizziness, and headache [see Drug Interactions ( 7.1) and Clinical Pharmacology ( 12.2)].
5.8 Combination with Other PDE5 Inhibitors or Erectile Dysfunction Therapies
The safety and efficacy of combinations of avanafil with other treatments for ED has not been studied. Therefore, the use of such combinations is not recommended.
5.9 Effects on Bleeding
The safety of avanafil is unknown in patients with bleeding disorders and patients with active peptic ulceration. In vitrostudies with human platelets indicate that avanafil potentiates the anti-aggregatory effect of sodium nitroprusside (a nitric oxide [NO] donor).
Close5.10 Counseling Patients about Sexually Transmitted Diseases
The use of avanafil offers no protection against sexually transmitted diseases. Counseling patients about the protective measures necessary to guard against sexually transmitted diseases, including Human Immunodeficiency Virus (HIV), should be considered.
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Avanafil was administered to 2215 men during clinical trials. In trials of avanafil for use as needed, a total of 493 patients were exposed for greater than or equal to 6 months, and 153 patients were treated for greater than or equal to 12 months.
In three randomized, double-blind, placebo-controlled trials lasting up to 3 months in duration, the mean age of patients was 56.4 years (range from 23 to 88 years). 83.9% of patients were White, 13.8% were Black, 1.4% Asian, and <1% Hispanic. 41.1% were current or previous smokers. 30.6% had diabetes mellitus.
The discontinuation rate due to adverse reactions for patients treated with avanafil 50 mg, 100 mg, or 200 mg was 1.4%, 2%, and 2%, respectively, compared to 1.7% for placebo-treated patients.
Table 1 presents the adverse reactions reported when avanafil was taken as recommended (on an as-needed basis) from these 3 clinical trials.
Table 1: Adverse Reactions Reported by Greater Than or Equal to 2% of Patients Treated with Avanafil From 3 Placebo-Controlled Clinical Trials Lasting 3 Months for Avanafil Use as Needed
Adverse Reaction
Placebo
(N = 349)
Avanafil
50 mg
(N = 217)
Avanafil
100 mg
(N = 349)
Avanafil
200 mg
(N = 352)
Headache
1.7%
5.1%
6.9%
10.5%
Flushing
0%
3.2%
4.3%
4%
Nasal congestion
1.1%
1.8%
2.9%
2%
Nasopharyngitis
2.9%
0.9%
2.6%
3.4%
Back pain
1.1%
3.2%
2%
1.1%
Adverse reactions reported by greater than or equal to 1%, but less than 2% of patients in any avanafil dose group, and greater than placebo included: upper respiratory infection (URI), bronchitis, influenza, sinusitis, sinus congestion, hypertension, dyspepsia, nausea, constipation, and rash.
In an open-label, long-term extension study of two of these randomized, double-blind, placebo-controlled trials, the total duration of treatment was up to 52 weeks. Among the 712 patients who participated in this open-label extension study, the mean age of the population was 56.4 years (range from 23 to 88 years). The discontinuation rate due to adverse reactions for patients treated with avanafil (50 mg, 100 mg, or 200 mg) was 2.8%.
In this extension trial, all eligible patients were initially assigned to avanafil 100 mg. At any point during the trial, patients could request to have their dose of avanafil increased to 200 mg or decreased to 50 mg based on their individual response to treatment. In total, 536 (approximately 75%) patients increased their dose to 200 mg and 5 (less than 1%) patients reduced their dose to 50 mg.
Table 2 presents the adverse reactions reported when avanafil was taken as recommended (on an as-needed basis) in this open-label extension trial.
Table 2: Adverse Reactions Reported by Greater Than or Equal to 2% of Patients Treated With Avanafil in an Open-Label Extension Trial
Adverse Reaction
Avanafil
(N = 711)
Headache
5.6%
Flushing
3.5%
Nasopharyngitis
3.4%
Nasal congestion
2.1%
Adverse reactions reported by greater than or equal to 1%, but less than 2% of patients in the open-label extension study included: upper respiratory infection (URI), influenza, sinusitis, bronchitis, dizziness, back pain, arthralgia, hypertension, and diarrhea.
The following events occurred in less than 1% of patients in the three placebo-controlled 3-month clinical trials and/or the open-label, long-term extension study lasting 12 months. A causal relationship to avanafil is uncertain. Excluded from this list are those events that were minor, those with no plausible relation to drug use and reports too imprecise to be meaningful.
Body as a whole— edema peripheral, fatigue
Cardiovascular— angina, unstable angina, deep vein thrombosis, palpitations
Digestive— gastritis, gastroesophageal reflux disease, hypoglycemia, blood glucose increased, alanine aminotransferase increased, oropharyngeal pain, stomach discomfort, vomiting
Musculoskeletal— muscle spasms, musculoskeletal pain, myalgia, pain in extremity
Nervous— depression, insomnia, somnolence, vertigo
Respiratory— cough, dyspnea exertional, epistaxis, wheezing
Skin and Appendages— pruritus
Urogenital — balanitis, erection increased, hematuria, nephrolithiasis, pollakiuria, urinary tract infection
In an additional, randomized, double-blind, placebo-controlled study lasting up to 3 months in 298 men who had undergone bilateral nerve-sparing radical prostatectomy for prostate cancer, the mean age of patients was 58.4 years (range 40 to 70). Table 3 presents the adverse reactions reported in this additional study.
Table 3: Adverse Reactions Reported by Greater than or Equal to 2% of Patients Treated with Avanafil in a Placebo-Controlled Clinical Trial Lasting 3 Months in Patients Who Underwent Bilateral Nerve-Sparing Radical Prostatectomy
Adverse Reaction
Placebo
(N = 100)
Avanafil
100 mg
(N = 99)
Avanafil
200 mg
(N = 99)
Headache
1%
8.1%
12.1%
Flushing
0%
5.1%
10.1%
Nasopharyngitis
0%
3%
5.1%
Upper respiratory infection
0%
2%
3%
Nasal congestion
1%
3%
1%
Back pain
1%
3%
2%
Electrocardiogram abnormal
0%
1%
3%
Dizziness
0%
1%
2%
A randomized, double-blind, placebo-controlled 2 months study was conducted in 435 subjects with a mean age of 58.2 years (range 24 to 86 years) to determine the time to onset of effect of avanafil, defined as the time to the first occurrence of an erection sufficient for sexual intercourse. Table 4 presents the adverse reactions occurring in ≥ 2% of subjects treated with avanafil.
Table 4: Adverse Reactions Reported by ≥ 2% of Patients Treated with Avanafil in a Placebo-Controlled Clinical Trial Lasting 2 Months to Determine the Time to Onset of Effect (Study 4)
Adverse Reaction
Placebo
(N = 143)
Avanafil
100 mg
(N = 146)
Avanafil
200 mg
(N = 146)
Headache
0.7%
1.4%
8.9%
Nasal congestion
0%
0.7%
4.1%
Gastroenteritis viral
0%
0%
2.1%
Across all trials with any avanafil dose, 1 subject reported a change in color vision.
Close6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of avanafil. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events have been chosen for inclusion either due to their seriousness, reporting frequency, lack of clear alternative causation, or a combination of these factors.
Cardiovascular and cerebrovascular:
Serious cardiovascular and cerebrovascular events, including myocardial infarction, sudden cardiac death, cerebrovascular accident, and subarachnoid hemorrhage, have been reported post-marketing in temporal association with the use of avanafil. All of these patients had preexisting cardiovascular risk factors. The occurrence of sexual activity was not stated in the majority of reports, although events were reported to occur both with and without sexual activity. It is not possible to determine whether these events are related directly to avanafil, to sexual activity, to the patient’s underlying cardiovascular disease, to a combination of these factors, or to other factors [see Warnings and Precautions ( 5.1) and Patient Counseling Information ( 17.2)].
Nervous:transient global amnesia
Special senses:
Ophthalmologic:vitreous detachment, visual impairment
Non-arteritic anterior ischemic optic neuropathy (NAION), a cause of decreased vision including permanent loss of vision, has been reported rarely post-marketing in temporal association with the use of phosphodiesterase type 5 (PDE5) inhibitors, such as avanafil. Most, but not all, of these patients had underlying anatomic or vascular risk factors for developing NAION, including but not necessarily limited to: low cup to disc ratio (“crowded disc”), age over 50, diabetes, hypertension, coronary artery disease, hyperlipidemia and smoking [see Warnings and Precautions ( 5.4)].
Otologic:Cases of sudden decrease or loss of hearing have been reported postmarketing in temporal association with the use of PDE5 inhibitors, such as avanafil. In some of the cases, medical conditions and other factors were reported that may have also played a role in the otologic adverse events. In many cases, medical follow-up information was limited. It is not possible to determine whether these reported events are related directly to the use of PDE5 inhibitors such as avanafil, to the patient’s underlying risk factors for hearing loss, a combination of these factors, or to other factors [see Warnings and Precautions ( 5.5) and Patient Counseling Information ( 17.6)]. -
7 DRUG INTERACTIONS7.1 Potential for Pharmacodynamic Interactions with Avanafil - Nitrates - Administration of avanafil to patients who are using any form of organic nitrate, is contraindicated. In a clinical ...
7.1 Potential for Pharmacodynamic Interactions with Avanafil
Nitrates
Administration of avanafil to patients who are using any form of organic nitrate, is contraindicated. In a clinical pharmacology trial, avanafil was shown to potentiate the hypotensive effect of nitrates. In a patient who has taken avanafil, where nitrate administration is deemed medically necessary in a life-threatening situation, at least 12 hours should elapse after the last dose of avanafil before nitrate administration is considered. In such circumstances, nitrates should only be administered under close medical supervision with appropriate hemodynamic monitoring [see Contraindications ( 4.1), Dosage and Administration ( 2.3), and Clinical Pharmacology ( 12.2)].
Alpha-Blockers
Caution is advised when PDE5 inhibitors are co-administered with alpha-blockers. PDE5 inhibitors, including avanafil, and alpha-adrenergic blocking agents are both vasodilators with blood pressure-lowering effects. When vasodilators are used in combination, an additive effect on blood pressure may be anticipated. In some patients, concomitant use of these two drug classes can lower blood pressure significantly leading to symptomatic hypotension (e.g., dizziness, lightheadedness, fainting) [see Warnings and Precautions ( 5.6), Dosage and Administration ( 2.3), and Clinical Pharmacology ( 12.2)].
Antihypertensives
PDE5 inhibitors, including avanafil, are mild systemic vasodilators. A clinical pharmacology trial was conducted to assess the effect of avanafil on the potentiation of the blood pressure-lowering effects of selected antihypertensive medications (amlodipine and enalapril). Additional reductions in blood pressure of 3 to 5 mmHg occurred following co-administration of a single 200 mg dose of avanafil with these agents compared with placebo [see Warnings and Precautions ( 5.6) and Clinical Pharmacology ( 12.2)].
Alcohol
Both alcohol and PDE5 inhibitors, including avanafil, act as vasodilators. When vasodilators are taken in combination, blood pressure-lowering effects of each individual compound may be increased. Substantial consumption of alcohol (e.g., greater than 3 units) in combination with avanafil can increase the potential for orthostatic signs and symptoms, including increase in heart rate, decrease in standing blood pressure, dizziness, and headache [see Clinical Pharmacology ( 12.2)].Close7.2 Potential for Other Drugs to Affect Avanafil
Avanafil is a substrate of and predominantly metabolized by CYP3A4. Studies have shown that drugs that inhibit CYP3A4 can increase avanafil exposure.
Strong CYP3A4 Inhibitors
Ketoconazole (400 mg daily), a selective and strong inhibitor of CYP3A4, increased avanafil 50 mg single-dose systemic exposure (AUC) and maximum concentration (C max)equal to 13-fold and 3-fold, respectively, and prolonged the half-life of avanafil to approximately 9 hours. Other potent inhibitors of CYP3A4 (e.g., itraconazole, clarithromycin, nefazadone, ritonavir, saquinavir, nelfinavir, indinavir, atanazavir and telithromycin) would be expected to have similar effects. Do not use avanafil in patients taking strong CYP3A4 inhibitors [see Warnings and Precautions ( 5.2) and Dosage and Administration ( 2.3)].
HIV Protease inhibitor— Ritonavir (600 mg twice daily), a strong CYP3A4 inhibitor, which also inhibits CYP2C9, increased avanafil 50 mg single-dose C maxand AUC equal to approximately 2-fold and 13-fold, and prolonged the half-life of avanafil to approximately 9 hours in healthy volunteers. Do not use avanafil in patients taking ritonavir.
Moderate CYP3A4 Inhibitors
Erythromycin (500 mg twice daily) increased avanafil 200 mg single-dose C maxand AUC equal to approximately 2-fold and 3-fold, respectively, and prolonged the half-life of avanafil to approximately 8 hours in healthy volunteers. Moderate CYP3A4 inhibitors (e.g., erythromycin, amprenavir, aprepitant, diltiazem, fluconazole, fosamprenavir, and verapamil) would be expected to have similar effects. Consequently, the maximum recommended dose of avanafil is 50 mg, not to exceed once every 24 hours for patients taking concomitant moderate CYP3A4 inhibitors [see Warnings and Precautions ( 5.2) and Drug Interactions ( 7.2)].
Although specific interactions have not been studied, other CYP3A4 inhibitors, including grapefruit juice are likely to increase avanafil exposure.
Weak CYP3A4 Inhibitors
No in vivodrug-drug interaction studies with weak CYP3A4 inhibitors were conducted.
CYP3A4 Substrate
When administered with avanafil 200 mg, amlodipine (5 mg daily) increased the C maxand AUC of avanafil by approximately 22% and 70%, respectively. The half-life of avanafil was prolonged to approximately 10 hrs. The C maxand AUC of amlodipine decreased by approximately 9% and 4%, respectively [see Dosage and Administration ( 2.3)].
Cytochrome P450 Inducers
The potential effect of CYP inducers on the pharmacokinetics of avanafil was not evaluated. The concomitant use of avanafil and CYP inducers is not recommended. -
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Avanafil is not indicated for use in females. There are no data with the use of avanafil in pregnant women to inform any drug-associated risks for ...
8.1 Pregnancy
Risk Summary
Avanafil is not indicated for use in females.
There are no data with the use of avanafil in pregnant women to inform any drug-associated risks for adverse developmental outcomes. In animal reproduction studies conducted in pregnant rats and rabbits, no adverse developmental outcomes were observed with oral administration of avanafil during organogenesis at exposures for total avanafil at approximately 8 and 6 times, respectively, the Maximum Recommended Human Dose (MRHD) of 200 mg based on AUC (see Data).
Data
Animal Data
In pregnant rats administered orally at 100, 300, or 1000 mg/kg/day from gestation days 6 to 17, no evidence of teratogenicity, embryotoxicity, or fetotoxicity was observed at up to 300 mg/kg/day. This dose is equivalent to exposures of approximately 8 times the exposure at the Maximum Recommended Human Dose (MRHD) of 200 mg based on AUCs for total avanafil. At the maternally toxic dose (1000 mg/kg/day), decreased fetal body weight occurred with no signs of teratogenicity. In pregnant rabbits administered orally at 30, 60, 120, or 240 mg/kg/day from gestation days 6 to 18, no teratogenicity was observed at exposures up to approximately 6 times the human exposure at the MRHD based on AUCs for total avanafil.
In a pre- and post-natal development study in rats given orally at 100, 300, or 600 mg/kg/day on gestation days 6 through lactation day 20, offspring growth and maturation were reduced when maternal rats were given avanafil doses greater than or equal to 300 mg/kg/day, resulting in exposures greater than or equal to 17 times the human exposure to total avanafil at the MRHD. There was no effect on reproductive performance of the maternal rats or offspring, or on the behavior of the offspring at up to the highest dose tested. The no observed adverse effect level (NOAEL) for developmental toxicity (100 mg/kg/day) was observed at exposures to total avanafil approximately 2-fold greater than the systemic exposure in humans at the MRHD.8.2 Lactation
Risk Summary
Avanafil is not indicated for use in females.
There is no information on the presence of avanafil and/or its metabolites in human or animal milk, the effects on the breastfed child, or the effects on milk production.8.3 Females and Males of Reproductive Potential
Infertility
There have been no studies evaluating the effect of avanafil on fertility in men [see Clinical Pharmacology ( 12.2)]
Based on studies in animals, decreased fertility, abnormal sperm motility and morphology, and altered estrous cycles were observed in rats. The abnormal sperm findings were reversible at the end of a 9-week drug-free period [see Nonclinical Toxicology ( 13.1)].8.4 Pediatric Use
Avanafil is not indicated for use in pediatric patients. Safety and efficacy in patients below the age of 18 years has not been established.
8.5 Geriatric Use
Of the total number of subjects in clinical studies of avanafil, approximately 23% were 65 and over. No overall differences in efficacy and safety were observed between subjects over 65 years of age compared to younger subjects; therefore, no dose adjustment is warranted based on age alone. However, a greater sensitivity to medication in some older individuals should be considered [see Clinical Pharmacology ( 12.3)].
8.6 Renal Impairment
In a clinical pharmacology trial using single 200 mg doses of avanafil, avanafil exposure (AUC or C max)in normal subjects was comparable to patients with mild (creatinine clearance greater than or equal to 60 to less than 90 mL/min) or moderate (creatinine clearance greater than or equal to 30 to less than 60 mL/min) renal impairment. No dose adjustment is necessary for patients with mild to moderate renal impairment (creatinine clearance greater than or equal to 30 to less than 90 mL/min). The pharmacokinetics of avanafil in patients with severe renal disease or on renal dialysis has not been studied; do not use avanafil in such patients [see Clinical Pharmacology ( 12.3)].
Close8.7 Hepatic Impairment
In a clinical pharmacology trial, avanafil AUC and C maxin patients with mild hepatic impairment (Child-Pugh Class A) was comparable to that in healthy subjects when a dose of 200 mg was administered. Avanafil C maxwas approximately 51% lower and AUC was 11% higher in patients with moderate hepatic impairment (Child Pugh Class B) compared to subjects with normal hepatic function. No dose adjustment is necessary for patients with mild to moderate hepatic impairment (Child Pugh Class A or B). The pharmacokinetics of avanafil in patients with severe hepatic disease has not been studied; do not use avanafil in such patients [see Clinical Pharmacology ( 12.3)].
-
10 OVERDOSAGESingle doses up to 800 mg have been given to healthy subjects, and multiple doses up to 300 mg have been given to patients. In cases of overdose, standard supportive measures should be adopted ...
Single doses up to 800 mg have been given to healthy subjects, and multiple doses up to 300 mg have been given to patients. In cases of overdose, standard supportive measures should be adopted as required. Renal dialysis is not expected to accelerate clearance because avanafil is highly bound to plasma proteins and is not significantly eliminated in the urine.
Close -
11 DESCRIPTIONAvanafil is a selective inhibitor of cGMP-specific PDE5. Avanafil is designated chemically as (S)-4-[(3-Chloro-4-methoxybenzyl)amino]-2-[2-(hydroxymethyl)-1-pyrrolidinyl] ...
Avanafil is a selective inhibitor of cGMP-specific PDE5.
Avanafil is designated chemically as (S)-4-[(3-Chloro-4-methoxybenzyl)amino]-2-[2-(hydroxymethyl)-1-pyrrolidinyl]- N-(2pyrimidinylmethyl)-5-pyrimidinecarboxamide and has the following structural formula:

Avanafil occurs as an off-white to white powder, molecular formula C 23H 26ClN 7O 3and molecular weight of 483.95 and is slightly soluble in methanol, ethanol and insoluble in water. Avanafil tablets, for oral administration, is supplied as oval, white to off white tablets containing 50 mg, 100 mg, or 200 mg avanafil. In addition to the active ingredient, avanafil, each tablet contains the following inactive ingredients: fumaric acid, hydroxyl propyl cellulose, low- substituted hydroxyl propyl cellulose, magnesium stearate, mannitol and sodium bicarbonate.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The physiologic mechanism of erection of the penis involves release of nitric oxide (NO) in the corpus cavernosum during sexual stimulation. NO then activates the ...
12.1 Mechanism of Action
The physiologic mechanism of erection of the penis involves release of nitric oxide (NO) in the corpus cavernosum during sexual stimulation. NO then activates the enzyme guanylate cyclase, which results in increased levels of cGMP, producing smooth muscle relaxation in the corpus cavernosum and allowing inflow of blood. Avanafil has no direct relaxant effect on isolated human corpus cavernosum, but enhances the effect of NO by inhibiting PDE5, which is responsible for degradation of cGMP in the corpus cavernosum. Because sexual stimulation is required to initiate the local release of nitric oxide, the inhibition of PDE5 has no effect in the absence of sexual stimulation.
Studies in vitrohave shown that avanafil is selective for PDE5. Its effect is more potent on PDE5 than on other known phosphodiesterases (greater than 100-fold for PDE6; greater than 1,000-fold for PDE4, PDE8 and PDE10; greater than 5,000-fold for PDE2 and PDE7; greater than 10,000-fold for PDE1, PDE3, PDE9, and PDE11). Avanafil is greater than 100-fold more potent for PDE5 than PDE6, which is found in the retina and is responsible for phototransduction. In addition to human corpus cavernosum smooth muscle, PDE5 is also found in other tissues including platelets, vascular and visceral smooth muscle, and skeletal muscle, brain, heart, liver, kidney, lung, pancreas, prostate, bladder, testis, and seminal vesicle. The inhibition of PDE5 in these tissues by avanafil may be the basis for the enhanced platelet anti-aggregatory activity of NO observed in vitroand peripheral vasodilatation in vivo.12.2 Pharmacodynamics
Effects of Avanafil on Erectile Response
In a single-blind, placebo-controlled, single-dose trial of 82 patients with either organic and/or psychogenic ED, visual sexual stimulation resulted in improved erections after avanafil administration compared to placebo, as assessed by an objective measurement of hardness and duration of erections (RigiScan ®). Efficacy was assessed by RigiScan at discrete time intervals ranging from 20 to 40 minutes after dosing to 100 to 120 minutes after dosing.
Effects of Avanafil on Blood Pressure
Single oral doses of avanafil (200 mg) administered to healthy male volunteers resulted in mean changes from baseline in systolic/diastolic blood pressure of -5.3/-3.7 mmHg at 1 hour after dosing, compared to mean changes from baseline in the placebo group of 2.7/-0.4 mmHg. The reductions in systolic/diastolic blood pressure at 1 hour after dosing of avanafil 200 mg compared to placebo were 8.0/3.3 mmHg.
Figure 1: Median Change from Baseline in Sitting Systolic Blood Pressure, Healthy Volunteers Day 4
Effects on Cardiac Electrophysiology
The effect of single 100 or 800 mg doses of avanafil on the QT interval were evaluated in a randomized, double-blind, placebo, and active (moxifloxacin) –controlled crossover study in 52 healthy male subjects aged 18 to 45 years. There were no significant effects of the 100 mg dose. The mean QTc (Fridericia QT correction) for avanafil 800 mg, relative to placebo was 9.4 milliseconds (two-sided 90% CI=7.2, 11.6). An 800 mg dose of avanafil (4 times the highest recommended dose) was chosen because this dose yields exposures greater than those observed upon co-administration of avanafil with strong CYP3A4 inhibitors. A double-blind, randomized, placebo- and active-controlled (moxifloxacin), thorough QT/QTc trial of avanafil (100 and 800 mg) in healthy male subjects demonstrated that avanafil did not cause any significant changes in QTc interval or ventricular repolarization.
Effects of Avanafil on Blood Pressure When Administered with Nitrates
In a clinical pharmacology trial, a single dose of avanafil 200 mg was shown to potentiate the hypotensive effect of nitrates. The use of avanafil in patients taking any form of nitrates is contraindicated [see Contraindications ( 4.1)].
A trial was conducted to assess the degree of interaction between nitroglycerin and avanafil, should nitroglycerin be required in an emergency situation after avanafil was taken. This was a single-center, double-blind, randomized, 3-way crossover trial of healthy males from 30 to 60 years of age. Subjects were divided among 5 trial groups with the trial group being determined by the time interval between treatment with trial drug and glyceryl trinitrate administration. Subjects were assigned to trial groups sequentially and hemodynamic results from the previous group were reviewed for serious adverse events (SAEs) before the next group received treatment. Each subject was dosed with all 3 study drugs (avanafil 200 mg, sildenafil citrate 100 mg, and placebo) in random order. Subjects were administered a single dose of 0.4 mg sublingual nitroglycerin (NTG) at pre-specified time points, following their dose of trial drug (0.5, 1, 4, 8 or 12 hours). Overall, 14 (15%) subjects treated with placebo and 28 (28%) subjects treated with avanafil, had clinically significant decreases in standing SBP, defined as greater than or equal to 30 mmHg decrease in SBP, after glyceryl trinitrate administration. Mean maximum decreases are shown in Table 5.
Table 5: Mean Maximum Decreases from Baseline in Sitting and Standing Systolic Blood Pressure/Diastolic Blood Pressure (mmHg) following Placebo or 200 mg Avanafil with 0.4 mg sublingual nitroglycerin
Placebo with nitroglycerin
Sitting
Standing
13.4/11.8
21.1/16.5
Avanafil with nitroglycerin
Sitting
Standing
21.6/18.2
28.0/23.5
Like other PDE5 inhibitors, avanafil administration with nitrates is contraindicated. In a patient who has taken avanafil, where nitrate administration is deemed medically necessary in a life threatening situation, at least 12 hours should elapse after the last dose of avanafil before nitrate administration is considered. In such circumstances, nitrates should still only be administered under close medical supervision with appropriate hemodynamic monitoring [see Contraindications ( 4.1)] .
Effects of Avanafil on Blood Pressure When Administered with Alpha-Blockers
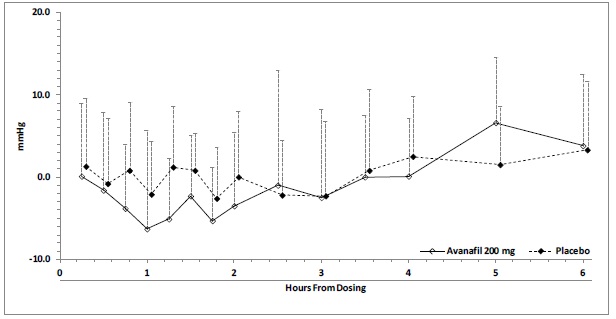
A single-center, randomized, double-blinded, placebo-controlled, two-period crossover trial was conducted to investigate the potential interaction of avanafil with alpha-blocker agents in healthy male subjects which consisted of two cohorts:
Cohort A (N=24): Subjects received oral doses of doxazosin once daily in the morning at 1 mg for 1 day (Day 1), 2 mg for 2 days (Days 2 to 3), 4 mg for 4 days (Days 4 to 7), and 8 mg for 11 days (Days 8 to 18). On Days 15 and 18, the subjects also received a single oral dose of either 200 mg avanafil or placebo, according to the treatment randomization code. The avanafil or placebo doses were administered 1.3 hours after the doxazosin administration on Days 15 and 18. The co-administration was designed so that doxazosin (T max~2 hours) and avanafil (T max~0.7 hours) would reach their peak plasma concentrations at the same time.
Cohort B (N=24): Subjects received 0.4 mg daily oral doses of tamsulosin in the morning for 11 consecutive days (Days 1 to 11). On Days 8 and 11, the subjects also received a single oral dose of either 200 mg avanafil or placebo, according to the treatment randomization code. The avanafil or placebo doses were administered 3.3 hours after the tamsulosin administration on Days 8 and 11. The co-administration was designed so that tamsulosin (T max~4 hours) and avanafil (T max~0.7 hours) would reach their peak plasma concentrations at the same time.
Supine and sitting BP and pulse rate measurements were recorded before and after avanafil or placebo dosing.
A total of seven subjects in Cohort A (doxazosin) experienced potentially clinically important absolute values or changes from baseline in standing SBP or DBP. Three subjects experienced standing SBP values less than 85 mmHg. One subject experienced a decrease from baseline in standing SBP greater than 30 mmHg following avanafil. Two subjects experienced standing DBP values less than 45 mmHg following avanafil. Four subjects experienced decreases from baseline in standing DBP greater than 20 mmHg following avanafil. One subject experienced such decreases following placebo. There were no severe adverse events related to hypotension reported during the trial. There were no cases of syncope.
A total of five subjects in Cohort B (tamsulosin) experienced potentially clinically important absolute values or changes from baseline in standing SBP or DBP. Two subjects experienced standing SBP values less than 85 mmHg following avanafil. One subject experienced a decrease from baseline in standing SBP greater than 30 mmHg following avanafil. Two subjects experienced standing DBP values less than 45 mmHg following avanafil. Four subjects experienced decreases from baseline in standing DBP greater than 20 mmHg following avanafil; one subject experienced such decreases following placebo. There were no severe adverse events related to hypotension reported during the trial. There were no cases of syncope.
Table 6 presents the placebo-subtracted mean maximum decreases from baseline (95% CI) in systolic blood pressure results for the 24 subjects who received avanafil 200 mg and matching placebo.
Table 6: Placebo-Subtracted Mean (95% CI) Maximum Decreases from Baseline in Standing and Supine Systolic Blood Pressure (mmHg) with 200 mg Avanafil
Doxazosin
Supine
Standing
-6 (-9.1, -2.9)
-2.5 (-6.5, 1.5)
Tamsulosin
Supine
Standing
-3.1 (-6.4, 0.1)
-3.6 (-8.1, 0.9)
Blood pressure effects (standing SBP) in normotensive men on stable dose doxazosin (8 mg) following administration of avanafil 200 mg or placebo, are shown in Figure 2. Blood pressure effects (standing SBP) in normotensive men on stable dose tamsulosin (0.4 mg) following administration of avanafil 200 mg or placebo are shown in Figure 3.
Figure 2: Mean (SD) Change From Baseline in Standing Systolic Blood Pressure Over Time Following Administration of a Single Dose 200 mg Dose of Avanafil with Doxazosin
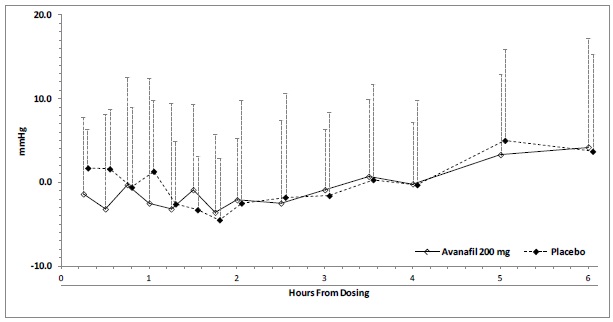
Figure 3: Mean (SD) Change From Baseline in Standing Systolic Blood Pressure Over Time Following Administration of a Single Dose 200 mg Dose of Avanafil with Tamsulosin
Effects of Avanafil on Blood Pressure When Administered with Enalapril
A trial was conducted to assess the interaction of enalapril (20 mg daily) and avanafil 200 mg. Single doses of 200 mg avanafil co-administered with enalapril caused a mean maximum decrease in supine systolic/diastolic blood pressure of 1.8/3.5 mmHg (compared to placebo), accompanied by a mean maximum increase in pulse rate of 1.0 bpm.
Effects of Avanafil on Blood Pressure When Administered with Amlodipine
A trial was conducted to assess the interaction of amlodipine (5 mg daily) and avanafil 200 mg. Single doses of 200 mg avanafil co-administered with amlodipine caused a mean maximum decrease in supine systolic blood pressure of 1.2 mmHg (compared to placebo), accompanied by a mean maximum increase in pulse rate of 1.0 bpm; the mean maximum decrease in diastolic blood pressure was less than that observed in the placebo group. There was no effect of avanafil on amlodipine plasma concentrations. Concomitant amlodipine was associated with 22% and 70% increases in avanafil C maxand AUC, respectively.
Effects of Avanafil on Blood Pressure When Administered with Alcohol
Alcohol and PDE5 inhibitors, including avanafil, are mild systemic vasodilators. The interaction of avanafil with alcohol was evaluated in a clinical pharmacology trial. Alcohol was administered at a dose of 0.5 g/kg, which is equivalent to approximately 3 ounces of 80-proof vodka in a 70-kg male, and avanafil was administered at a dose of 200 mg. All patients consumed the entire alcohol dose within 15 minutes of starting. Blood alcohol levels of 0.057% were confirmed. There were no reports of orthostatic hypotension or dizziness. Additional maximum supine systolic/diastolic blood pressure decreases of 3.5/4.5 mmHg and additional maximum pulse rate increase of 9.3 bpm were observed when avanafil was taken with alcohol compared to alcohol alone. Avanafil did not affect alcohol plasma concentrations.
Effects of Avanafil on Spermatogenesis
The effect of avanafil on spermatogenesis was assessed in 181 healthy male volunteers who received avanafil 100 mg or placebo daily for 26 weeks. The results of this randomized, double-blind, placebo-controlled study in 137 subjects who completed the study through Week 26 and provided 2 semen samples at baseline and at Week 26 showed no adverse effects of avanafil on sperm concentration, total sperm count, sperm motility, sperm normal morphology, and semen volume.
Effects of Avanafil on Vision
Single oral doses of Type 5 phosphodiesterase inhibitors have demonstrated transient dose-related impairment of color discrimination (blue/green), using the Farnsworth-Munsell 100-hue test, with peak effects near the time of peak plasma levels. This finding is consistent with the inhibition of PDE6, which is involved in phototransduction in the retina.Close12.3 Pharmacokinetics
Mean avanafil plasma concentrations measured after the administration of a single oral dose of 50 or 200 mg to healthy male volunteers are depicted in Figure 4. The pharmacokinetics of avanafil are dose proportional from 12.5 to 600 mg.
Figure 4: Plasma Avanafil Concentrations (mean ± SD) Following a Single 50 mg or 200 mg Avanafil Dose
Absorption and Distribution
Avanafil is rapidly absorbed after oral administration, with a median T maxof 30 to 45 minutes in the fasted state. When avanafil (200 mg) is taken with a high fat meal, the rate of absorption is reduced, with a mean delay in T maxof 1.12 to 1.25 hours and a mean reduction in C maxof 39% (200 mg). There was an approximate 3.8% decrease in AUC. The small changes in avanafil C maxand AUC are considered of minimal clinical significance; therefore, avanafil may be administered with or without food. The mean accumulation ratio is approximately 1.2. Avanafil is approximately 99% bound to plasma proteins. Protein binding is independent of total drug concentrations, age, renal and hepatic function.
Based upon measurements of avanafil in semen of healthy volunteers 45 to 90 minutes after dosing, less than 0.0002% of the administered dose appeared in the semen of patients.
Metabolism and Excretion
Avanafil is cleared predominantly by hepatic metabolism, mainly by the CYP3A4 enzyme and to a minor extent by CYP2C isoform. The plasma concentrations of the major circulating metabolites, M4 and M16, are approximately 23% and 29% that of the parent compound, respectively. The M4 metabolite has an in vitroinhibitory potency for PDE5 18% of that of avanafil and M4 accounts for approximately 4% of the pharmacologic activity of avanafil. The M16 metabolite was inactive against PDE5.
Avanafil was extensively metabolized in humans. After oral administration, avanafil is excreted as metabolites predominantly in the feces (approximately 62% of administered oral dose) and to a lesser extent in the urine (approximately 21% of the administered oral dose). Avanafil has a terminal elimination half-life of approximately 5 hours.Geriatric
The pharmacokinetics of a single 200 mg avanafil administered to fourteen healthy elderly male volunteers (65 to 80 years) and eighteen healthy younger male volunteers (18 to 43 years of age) were compared. AUC 0 to infincreased by 6.8% and C maxdecreased by 2.1% in the elderly group, compared to the younger group. However, greater sensitivity to medications in some older individuals should be considered [see Use in Specific Populations ( 8.5)].
Renal Impairment
The pharmacokinetics of a single 200 mg avanafil administered to nine patients with mild (creatinine clearance greater than or equal to 60 and less than 90 mL/min) and to ten patients with moderate (creatinine clearance greater than or equal to 30 to less than 60 mL/min) renal impairment were evaluated. AUC 0 to infdecreased by 2.9% and C maxincreased by 2.8% in patients with mild renal impairment, compared to healthy volunteers with normal renal function. AUC 0 to infincreased by 9.1% and C maxdecreased by 2.8% in patients with moderate renal impairment, compared to healthy volunteers with normal renal function. There is no data available for subjects with severe renal insufficiency or end-stage renal disease on hemodialysis [see Use in Specific Populations ( 8.6)].
Hepatic Impairment
The pharmacokinetics of a single 200 mg avanafil administered to eight patients with mild hepatic impairment (Child-Pugh A) and eight patients with moderate hepatic impairment (Child-Pugh B) were evaluated. AUC 0 to infincreased by 3.8% and C maxdecreased by 2.7% in patients with mild hepatic impairment, compared to healthy volunteers with normal hepatic function. AUC 0 to infincreased by 11.2% and C maxdecreased by 51% in patients with moderate hepatic impairment, compared to healthy volunteers with normal hepatic function. There is no data available for subjects with severe hepatic impairment (Child-Pugh Class C) [see Use in Specific Populations ( 8.7)].
Drug Interaction Studies
Clinical Studies
Effect of CYP3A4 Inhibitors on Avanafil:Strong and moderate CYP3A4 inhibitors increase plasma concentrations of avanafil. The effect of strong CYP3A4 inhibitors, ketoconazole and ritonavir, and moderate CYP3A4 inhibitor, erythromycin, on avanafil pharmacokinetics was studied in an open-label, randomized, one-sequence crossover, three-way parallel study.
Strong CYP3A4 Inhibitors
Fifteen healthy male volunteers received 400 mg ketoconazole (2 tablets containing 200 mg ketoconazole) once daily for 5 days (Days 2 to 6) and a single 50 mg avanafil on Days 1 and 6. Twenty-four hour pharmacokinetics of avanafil on Days 1 and 6 were compared. Co-administration with the strong CYP3A4 inhibitor ketoconazole resulted in an approximate 13-fold increase in AUC 0 to infand 3.1-fold increase in C max. Fourteen healthy male volunteers received 300 mg ritonavir (3 tablets containing 100 mg ritonavir) twice daily for 1 day (Day 2), 400 mg twice daily for 1 day (Day 3), 600 mg twice daily for 5 days (Days 4 to 8), and a single 50 mg avanafil on Days 1 and 8. Twenty-four hour pharmacokinetics of avanafil on Days 1 and 8 were compared. Co-administration with the strong CYP3A4 inhibitor ritonavir resulted in an approximate 13-fold increase in AUC 0 to infand 2.4-fold increase in C maxof avanafil.
Moderate CYP3A4 Inhibitors
Fifteen healthy male volunteers received 500 mg erythromycin (2 tablets containing 250 mg erythromycin) every 12 hrs for 5 days (Days 2 to 6) and a single 200 mg avanafil (2 tablets containing 100 mg avanafil) on Days 1 and 6. Twenty-four hour pharmacokinetics of avanafil on Days 1 and 6 were compared. Co-administration with the moderate CYP3A4 inhibitor erythromycin resulted in an approximate 3.6-fold increase in AUC 0 to infand 2-fold increase in C maxof avanafil.
Effect of Avanafil on Other Drugs:
Warfarin
The effect of avanafil on warfarin pharmacokinetics and pharmacodynamics was evaluated in a double-blind, randomized, placebo-controlled, two-way crossover study. Twenty-four healthy male volunteers were randomized to receive either 200 mg avanafil or matching placebo for 9 days. On Day 3 of each period, volunteers received a single 25 mg warfarin. Pharmacokinetics of R- and S-warfarin, PT, and INR prior to warfarin dosing and up to 168 hrs after warfarin administration were compared. Platelet aggregation prior to warfarin dosing and up to 24 hrs after warfarin administration were compared. PT, INR, and platelet aggregation did not change with avanafil administration: 23.1 sec, 2.2, and 75.5%, respectively. Co-administration with avanafil resulted in an approximate 1.6% increase in AUC 0 to infand 5.2% decrease in C maxof S-warfarin.
Omeprazole, Rosiglitazone, and Desipramine
The effect of avanafil on the pharmacokinetics of omeprazole (a CYP2C19 substrate), rosiglitazone (a CYP2C8 substrate), and desipramine (a CYP2D6 substrate) was evaluated in an open-label, three cohort, crossover study. Nineteen healthy male volunteers received a single 40 mg omeprazole delayed-release capsule once daily for 8 days (Days 1 to 8), and a single 200 mg avanafil on Day 8. Twelve hour pharmacokinetics of omeprazole on Days 7 and 8 were compared. Co-administration with avanafil resulted in an approximate 5.9% increase in AUC 0 to infand 8.6% increase in C maxof omeprazole. Twenty healthy male volunteers received a single 8 mg rosiglitazone tablet then a single 200 mg avanafil. Twenty-four hour pharmacokinetics of rosiglitazone with and without avanafil were compared. Co-administration with avanafil resulted in an approximate 2% increase in AUC 0 to infand 14% decrease in C maxof rosiglitazone. Twenty healthy male volunteers received a single 50 mg desipramine tablet then a single 200 mg avanafil tablet 2 hours after desipramine. Ninety-six hour pharmacokinetics of desipramine with and without avanafil were compared. Co-administration with avanafil resulted in an approximate 5.7% increase in AUC 0 to infand 5.2% increase in C maxof desipramine.
In vitro studies
Avanafil had no effect on CYP1A1/2, 2A6, 2B6 and 2E1 (IC50 greater than 100 micromolar) and weak inhibitory effects toward other isoforms (CYP2C8, 2C9, 2C19, 2D6, 3A4). Major circulating metabolites of avanafil (M4 and M16) had no effect on CYPs 1A, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1 and 3A4. Avanafil and its metabolites (M4 and M16) are unlikely to cause clinically significant inhibition of CYPs 1A, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1 or 3A4. -
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility - Carcinogenesis - Avanafil was not carcinogenic to CD-1 mice when administered daily at doses of 100, 200, or 600 mg/kg/day ...
13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility
Carcinogenesis
Avanafil was not carcinogenic to CD-1 mice when administered daily at doses of 100, 200, or 600 mg/kg/day orally by gavage for at least 98 weeks (approximately 11 times the MRHD on an AUC basis) or to Sprague Dawley rats when administered daily at doses of 100, 300, or 1000 mg/kg/day orally by gavage for at least 100 weeks (approximately 8 times for males and 34 times for females above the MRHD on an AUC basis).
Mutagenesis
Avanafil was not genotoxic in a series of tests. Avanafil was not mutagenic in Ames assays. Avanafil was not clastogenic in chromosome aberration assays using Chinese hamster ovary and lung cells, or in vivoin the mouse micronucleus assay. Avanafil did not affect DNA repair when tested in the rat unscheduled DNA synthesis assay.
Impairment of Fertility
In a rat fertility and early embryonic development study administered 100, 300, or 1000 mg/kg/day for 28 days prior to pairing and continued until euthanasia for males, and 14 days prior to pairing to gestation day 7 for females, a decrease in fertility, no or reduced sperm motility, altered estrous cycles, and an increased percentage of abnormal sperm (broken sperm with detached heads) occurred at exposures in males approximately 11 times the human exposure at a dose of 200 mg. The altered sperm effects were reversible at the end of a 9-week drug-free period. Systemic exposure at the NOAEL (300 mg/kg/day) was comparable to the human AUC at the MRHD of 200 mg.Close13.2 Animal Toxicology and/or Pharmacology
Repeated oral administration of avanafil in multiple species resulted in signs of centrally-mediated toxicity including ataxia, tremor, convulsion, hypoactivity, recumbency, and/or prostration at doses resulting in exposures approximately 5 to 8 times the MRHD based on C maxand 8 to 30 times the MRHD based on AUC.
-
14 CLINICAL STUDIESAvanafil was evaluated in four randomized, double-blind, placebo-controlled, parallel trials of 2 to 3 months in duration. Avanafil was taken as needed at doses of 50 mg, 100 mg, and 200 mg ...
Avanafil was evaluated in four randomized, double-blind, placebo-controlled, parallel trials of 2 to 3 months in duration. Avanafil was taken as needed at doses of 50 mg, 100 mg, and 200 mg (Study 1) and 100 mg and 200 mg (Study 2 and Study 4). Patients were instructed to take 1 dose of study drug approximately 30 minutes (Study 1 and Study 2) or approximately 15 minutes (Study 4) prior to initiation of sexual activity. Food and alcohol intake was not restricted.
In addition, a subset of patients from 2 of these trials were enrolled into an open-label extension trial. In the open-label extension trial, all eligible patients were initially assigned to avanafil 100 mg. At any point during the trial, patients could request to have their dose of avanafil increased to 200 mg or decreased to 50 mg based on their individual response to treatment.
The 3 primary outcome measures in Studies 1, 2 and 3 were the erectile function domain of the International Index of Erectile Function (IIEF) and Questions 2 and 3 from Sexual Encounter Profile (SEP). The IIEF is a 4-week recall questionnaire that was administered at baseline and at 4-week intervals during treatment. The IIEF erectile function domain has a 30-point total score, where the higher scores reflect better erectile function. The SEP included diary-based measures of erectile function. Patients recorded information regarding each sexual attempt made throughout the trial. Question 2 of the SEP asks “Were you able to insert your penis into your partner’s vagina?” Question 3 of the SEP asks “Did your erection last long enough for you to have successful intercourse?”
In Study 4, the primary efficacy variable was the per-subject proportion of sexual attempts that had an erectogenic effect within approximately 15 minutes following dosing, where an erectogenic effect was defined as an erection sufficient for vaginal penetration and that enabled satisfactory completion of sexual intercourse.
Results are shown from the two, Phase 3, randomized, double-blind, placebo-controlled, parallel studies, one in the general ED population (Study 1), another in the diabetic population with ED (Study 2).
Results in the General ED Population (Study 1):
Avanafil was evaluated in 646 men with ED of various etiologies (organic, psychogenic, mixed), in a randomized, double-blinded, parallel, placebo controlled fixed dose trial of 3 months duration. The mean age was 55.7 years (range 23 to 88 years). The population was 85.6% White, 13.2% Black, 0.9% Asian, and 0.3% of other races. The mean duration of ED was approximately 6.5 years. Avanafil at doses of 50 mg, 100 mg, and 200 mg demonstrated statistically significant improvement in all 3 primary efficacy variables relative to placebo (see Table 7).
Table 7: Mean Change From Baseline for Primary Efficacy Variables in General ED Population (Study 1)
Placebo
(N=155)
Avanafil 50 mg (N=154)
Avanafil 100 mg (N=157)
Avanafil 200 mg (N=156)
IIEF EF Domain Score
Endpoint
15.3
18.1
20.9
22.2
Change from baseline †
2.9
5.4
8.3
9.5
p-value*
0.0014
<0.0001
<0.0001
Vaginal Penetration (SEP2)
Endpoint
53.8%
64.3%
73.9%
77.3%
Change from baseline †
7.1%
18.2%
27.2%
29.8%
p-value*
-
0.0009
<0.0001
<0.0001
Successful Intercourse (SEP3)
Endpoint
27%
41.3%
57.1%
57%
Change from baseline †
14.1%
27.8%
43.4%
44.2%
p-value*
-
0.0002
<0.0001
<0.0001
† least-squares estimate from ANCOVA model * comparison to placebo for change from baseline
Results in the ED Population with Diabetes Mellitus (Study 2)
Avanafil was evaluated in ED patients (n=390) with type 1 or type 2 diabetes mellitus in a randomized, double-blind, parallel, placebo-controlled fixed dose trial of 3 months in duration. The mean age was 58 years (range 30 to 78 years). The population was 80.5% White, 17.2% Black, 1.5% Asian, and 0.8% of other races. The mean duration of ED was approximately 6 years. In this trial, avanafil at doses of 100 mg and 200 mg demonstrated statistically significant improvement in all 3 primary efficacy variables as measured by the erectile function domain of the IIEF questionnaire; SEP2 and SEP3 (see Table 8).
Table 8: Mean Change From Baseline for Primary Efficacy Variables in ED Population with Diabetes Mellitus (Study 2)
Placebo
(N=127)
Avanafil 100 mg (N=126)
Avanafil 200 mg (N=126)
IIEF EF Domain Score
Endpoint
13.2
15.8
17.3
Change from baseline †
1.8
4.5
5.4
p-value*
-
0.0017
<0.0001
Vaginal Penetration (SEP2)
Endpoint
42%
54%
63.5%
Change from baseline †
7.5%
21.5%
25.9%
p-value*
-
0.0004
<0.0001
Successful Intercourse (SEP3)
Endpoint
20.5%
34.4%
40%
Change from baseline †
13.6%
28.7%
34%
p-value*
-
<0.0001
<0.0001
† least-squares estimate from ANCOVA model * comparison to placebo for change from baseline
Time to Onset of Effect (Study 4)Avanafil was evaluated in 440 subjects with ED including diabetics (16.4%) and subjects with severe ED (41.4%) in a randomized double-blind, parallel, placebo-controlled study of 2 months duration. The mean age was 58.2 years (range 24 to 86 years). The population was 75.7% White, 21.4% Black, 1.6% Asian, and 1.4% of other races. Subjects were encouraged to attempt intercourse approximately 15 minutes after dosing and used a stopwatch for measurement of time to onset of effect, defined as the time to the first occurrence of an erection sufficient for sexual intercourse.
Avanafil 100 mg and 200 mg demonstrated statistically significant improvements relative to placebo in the primary efficacy variable, percentage of all attempts resulting in an erection sufficient for penetration at approximately 15 minutes after dosing followed by successful intercourse (SEP3) (see Table 10).
Table 10: Percentage of All Attempts Resulting in an Erection Sufficient for Penetration at Approximately 15 minutes After Dosing Followed by Successful Intercourse (SEP3) During the 8-Week Treatment Period in the Time to Onset of Effect (Study 4)
Placebo (N=136)
Avanafil 100 mg (N=139)
Avanafil 200 mg (N=139)
Percentage of Successful Intercourse (SEP3)
Mean
14.9
25.9
29.1
Median
0
11.1
13.3
p-value*
-
0.001
<0.001
* comparison to placebo using rank-ANCOVA model.
Close -
16 HOW SUPPLIED/STORAGE AND HANDLINGAvanafil tablets, 50 mg are white to off white, oval shape, biconvex tablets debossed with ‘A41’ on one side and ‘H’ on the other side. They are supplied as - Bottle of 30 tablets ...
Avanafil tablets, 50 mg are white to off white, oval shape, biconvex tablets debossed with ‘A41’ on one side and ‘H’ on the other side. They are supplied as
Close
Bottle of 30 tablets NDC 31722-440-30
Bottle of 100 tablets NDC 31722-440-01
Avanafil tablets, 100 mg are white to off white, oval shape, biconvex tablets debossed with ‘A42’ on one side and ‘H’ on the other side. They are supplied as
Bottle of 30 tablets NDC 31722-441-30
Bottle of 100 tablets NDC 31722-441-01
Avanafil tablets, 200 mg are white to off white, oval shape, biconvex tablets debossed with ‘A43’ on one side and ‘H’ on the other side. They are supplied as
Bottle of 30 tablets NDC 31722-442-30
Bottle of 100 tablets NDC 31722-442-01
Store at controlled room temperature 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).
Protect from light [see USP Controlled Room Temperature]. -
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). 17.1 Nitrates - Physicians should discuss with patients the contraindication of avanafil tablets with ...
Advise the patient to read the FDA-approved patient labeling (Patient Information).
17.1 Nitrates
Physicians should discuss with patients the contraindication of avanafil tablets with regular and/or intermittent use of organic nitrates. Patients should be counseled that concomitant use of avanafil tablets with nitrates could cause blood pressure to suddenly drop to an unsafe level, resulting in dizziness, syncope, or even heart attack or stroke.
Physicians should discuss with patients the appropriate action in the event that they experience anginal chest pain requiring nitroglycerin following intake of avanafil tablets. In such a patient, who has taken avanafil tablets, where nitrate administration is deemed medically necessary in a life-threatening situation, at least 12 hours should elapse after the last dose of avanafil tablets before nitrate administration is considered. In such circumstances, nitrates should still only be administered under close medical supervision with appropriate hemodynamic monitoring. Patients who experience anginal chest pain after taking avanafil tablets should seek immediate medical attention [see Contraindications ( 4.1) and Warnings and Precautions ( 5.1)].17.2 Cardiovascular Considerations
Physicians should discuss with patients the potential cardiac risk of sexual activity in patients with preexisting cardiovascular risk factors. Patients who experience symptoms upon initiation of sexual activity should be advised to refrain from further sexual activity and should seek immediate medical attention [see Warnings and Precautions ( 5.1)].
17.3 Concomitant Use with Drugs Which Lower Blood Pressure
Physicians should advise patients of the potential for avanafil tablets to augment the blood pressure-lowering effect of alpha-blockers and other antihypertensive medications [see Warnings and Precautions ( 5.6), Drug Interactions ( 7.1), and Clinical Pharmacology ( 12.2)].
17.4 Potential for Drug Interactions
Patients should be advised to contact the prescribing physician if new medications that may interact with avanafil are prescribed by another healthcare provider.
17.5 Priapism
There have been rare reports of prolonged erections greater than 4 hours and priapism (painful erections greater than 6 hours in duration) for this class of compounds. Priapism, if not treated promptly, can result in irreversible damage to the erectile tissue. Physicians should advise patients who have an erection lasting greater than 4 hours, whether painful or not, to seek emergency medical attention.
17.6 Vision
Physicians should advise patients to stop use of all PDE5 inhibitors, including avanafil tablets, and seek medical attention in the event of a sudden loss of vision in one or both eyes. Such an event may be a sign of non-arteritic anterior ischemic optic neuropathy (NAION), a cause of decreased vision including permanent loss of vision that has been reported rarely in temporal association with the use of PDE5 inhibitors. Physicians should discuss with patients the increased risk of NAION in individuals who have already experienced NAION in one eye. Physicians should also discuss with patients the increased risk of NAION among the general population in patients with a “crowded” optic disc, although evidence is insufficient to support screening of prospective users of PDE5 inhibitor, including avanafil tablets, for these uncommon conditions [see Warnings and Precautions ( 5.4) and Adverse Reactions ( 6.2)].
17.7 Sudden Hearing Loss
Physicians should advise patients to stop taking PDE5 inhibitors, including avanafil tablets, and seek prompt medical attention in the event of sudden decrease or loss of hearing. Use of PDE5 inhibitors has been associated with sudden decrease or loss of hearing, which may be accompanied by tinnitus and dizziness. It is not possible to determine whether these events are related directly to the use of PDE5 inhibitors or to other factors [see Adverse Reactions ( 6)].
17.8 Alcohol
Patients should be made aware that both alcohol and PDE5 inhibitors including avanafil tablets act as mild vasodilators. When mild vasodilators are taken in combination, blood pressure-lowering effects of each individual compound may be increased. Therefore, physicians should inform patients that substantial consumption of alcohol (e.g., greater than 3 units) in combination with avanafil tablets can increase the potential for orthostatic signs and symptoms, including increase in heart rate, decrease in standing blood pressure, dizziness, and headache [see Warnings and Precautions ( 5.7) and Clinical Pharmacology ( 12.2)].
17.9 Sexually Transmitted Disease
The use of avanafil tablets offers no protection against sexually transmitted diseases. Counseling of patients about the protective measures necessary to guard against sexually transmitted diseases, including Human Immunodeficiency Virus (HIV) should be considered.
17.10 Recommended Administration
Physicians should discuss with patients the appropriate use of avanafil tablets and its anticipated benefits. It should be explained that sexual stimulation is required for an erection to occur after taking avanafil tablets. Patients should be counseled regarding the dosing of avanafil tablets. Inform patients that the recommended starting dose of avanafil tablets is 100 mg, taken as early as approximately 15 minutes before initiation of sexual activity. Based on efficacy and tolerability, the dose may be increased to 200 mg taken as early as approximately 15 minutes before sexual activity, or decreased to 50 mg taken approximately 30 minutes before sexual activity. The lowest dose that provides benefit should be used. Patients should be advised to contact their healthcare provider for dose modification.
Close17.11 Guanylate Cyclase (GC) Stimulators
Physicians should discuss with patients the contraindication of avanafil tablets with use of guanylate cyclase stimulators such as riociguat or vericiguat [see Contraindications ( 4.3)].

Manufactured for:
Camber Pharmaceuticals, Inc.
Piscataway, NJ 08854
by: HETERO TM
Hetero Labs Limited
Jeedimetla, Hyderabad - 500 055, India
Revised: 07/2024 -
Patient InformationAvanafil - (a VAN a fil) tablets, for oral use - What is the most important information I should know about avanafil tablets? Avanafil tablets can cause your ...
Avanafil
(a VAN a fil)
tablets, for oral use
What is the most important information I should know about avanafil tablets?
Avanafil tablets can cause your blood pressure to drop suddenly to an unsafe level if it is taken with certain other medicines.Do not take avanafil tablets if you take any medicines called nitrates. Nitrates are used to treat chest pain (angina). A sudden drop in blood pressure can cause you to feel dizzy, faint, or have a heart attack or stroke.
Do not take avanafil tablets if you take medicines called guanylate cyclase stimulators which include:
• Riociguat and vericiguat, medicine that treat pulmonary arterial hypertension and chronic-thromboembolic pulmonary hypertension.
Ask your healthcare provider or pharmacist if any of your medicines are nitrates or guanylate cyclase stimulators.
Tell all your healthcare providers that you take avanafil tablets.If you need emergency medical care for a heart problem, it will be important for your healthcare provider to know when you last took avanafil tablets.
Stop sexual activity and get medical help right away if you get symptoms such as chest pain, dizziness, or nausea during sex. Sexual activity can put an extra strain on your heart, especially if your heart is already weak from a heart attack or heart disease.
What are avanafil tablets?
Avanafil tablet is a prescription medicine used to treat erectile dysfunction (ED) in adult men.
Avanafil tablet is not for use in women or children.
It is not known if avanafil tablet is safe and effective in women or children under 18 years of age.
Do not take avanafil tablet if you:
• take medicines called nitrates.
• use street drugs called “poppers” such as amyl nitrite and butyl nitrite.
• are allergic to avanafil or any of the ingredients in avanafil tablet. See the end of this leaflet for a complete list of ingredients in avanafil tablet.
• take any medicines called guanylate cyclase stimulators.
Before you take avanafil tablet, tell your healthcare provider about all of your medical conditions, including if you:
• have or have had heart problems such as a heart attack, irregular heartbeat, angina, or heart failure.
• have had heart surgery within the last 6 months.
• have had a stroke.
• have low blood pressure, or high blood pressure that is not controlled.
• have a deformed penis shape.
• have had an erection that lasted for more than 4 hours.
• have problems with your blood cells such as sickle cell anemia, multiple myeloma, or leukemia.
• have retinitis pigmentosa, a rare genetic (runs in families) eye disease.
• have ever had severe vision loss, including an eye problem called non-arteritic anterior ischemic optic neuropathy (NAION).
• have had a sudden decrease or loss of hearing.
• have bleeding problems.
• have or have had stomach ulcers.
• have liver problems.
• have kidney problems or are having kidney dialysis.
Tell your healthcare provider about all the medicines you take,including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Avanafil tablet may affect the way other medicines work, and other medicines may affect the way avanafil tablet works causing side effects. Especially tell your healthcare provider if you take any of the following:
• medicines called nitrates (see What is the most important information I should know about avanafil tablets?)
• medicines called guanylate cyclase stimulators, such a riociguat or vericiguat (see What is the most important information I should know about avanafil tablets?)
• medicines called HIV protease inhibitors.
• some types of oral antifungal medicines, such as ketoconazole, and itraconozale.
• some types of antibiotics, such as clarithromycin, telithromycin, or erythromycin.
• medicines called alpha blockers. These include terazosin, tamsulosin HCl, doxazosin, prazosin HCl, alfuzosin HCl, dutasteride and tamsulosin HCl, or silodosin. Alpha-blockers are sometimes prescribed for prostate problems or high blood pressure. In some patients, the use of avanafil tablet with alpha-blockers can lead to a drop in blood pressure or to fainting.
• other medicines that treat high blood pressure.
• other medicines or treatments for ED.
Ask your healthcare provider or pharmacist for a list of these medicines, if you are not sure.
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
How should I take avanafil tablets?
• Take avanafil tablets exactly as your healthcare provider tells you to take it.
• Your healthcare provider will tell you how much avanafil tablets to take and when to take it.
• Take avanafil tablets 100 mg or 200 mg as early as 15 minutes before sexual activity or
• Take avanafil tablets 50 mg as early as 30 minutes before sexual activity.
• Do not take avanafil tablets more than 1 time a day.
• Some form of sexual stimulation is needed for an erection to happen after taking avanafil tablets.
• Your healthcare provider may change your dose if needed.
• You should take the lowest dose of avanafil tablets that works for you. You and your healthcare provider should decide about the lowest dose of avanafil tablets that works for you.
• Avanafil tablets may be taken with or without food.
• If you take too much avanafil, call your healthcare provider or go to the nearest hospital emergency room right away.
What should I avoid while taking avanafil tablets?
• Do notdrink too much alcohol when taking avanafil tablets (for example, 3 glasses of wine, or 3 shots of whiskey). Drinking too much alcohol when taking avanafil tablets can increase your chances of increasing your heart rate, lowering your blood pressure, dizziness or getting a headache.
What are the possible side effects of avanafil tablets?
Avanafil tablets can cause serious side effects, including:
• See “What is the most important information I should know about avanafil tablets?”
• an erection that will not go away (priapism).If you have an erection that lasts more than 4 hours, get medical help right away. If it is not treated right away, priapism can permanently damage your penis.
• sudden vision loss in 1 or both eyes.Sudden vision loss in 1 or both eyes can be a sign of a serious eye problem called non-arteritic anterior ischemic optic neuropathy (NAION). It is uncertain whether PDE5 inhibitors such as avanafil tablets, directly cause vision loss. Stop taking avanafil tablets and call your healthcare provider right away if you have sudden vision loss in 1 or both eyes.
• sudden hearing decrease or hearing loss.Some people may also have ringing in their ears (tinnitus) or dizziness.
The most common side effects of avanafil tablets are:
• headache
• flushing
• stuffy or runny nose
• sore throat
• back pain
These are not all of the possible side effects of avanafil tablets.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store avanafil tablets?
• Store avanafil tablets at controlled room temperature 77°F (25°C).
• Keep avanafil tablets out of the light.
Keep avanafil tablets and all medicines out of the reach of children.
General information about the safe and effective use of avanafil tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use avanafil tablets for a condition for which it was not prescribed. Do not give avanafil tablets to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about avanafil tablets that is written for health professionals.
What are the ingredients in avanafil tablets?
Active ingredient:avanafil
Inactive ingredients:fumaric acid, hydroxyl propyl cellulose, low-substituted hydroxyl propyl cellulose, magnesium stearate, mannitol and sodium bicarbonate.
All brand names listed are the registered trademarks of their respective owners and are not trademarks of Hetero Labs Limited.
For more information, call 1-866-495-1995.

Manufactured for: by:
Camber Pharmaceuticals, Inc.
Piscataway, NJ 08854
by: HETERO TM
Hetero Labs Limited
Jeedimetla, Hyderabad - 500 055, India
This Patient Information has been approved by the U.S. Food and Drug Administration.
Close
Revised: 07/2024 -
PACKAGE LABEL.PRINCIPAL DISPLAY PANELAvanafil tablets 50 mg-30s label - Avanafil tablets 100 mg-30s label - Avanafil tablets 200 mg-30s label
Avanafil tablets 50 mg-30s label

Avanafil tablets 100 mg-30s label

Avanafil tablets 200 mg-30s label
Close
-
INGREDIENTS AND APPEARANCEProduct Information
AVANAFIL avanafil tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:31722-440 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVANAFIL (UNII: DR5S136IVO) (AVANAFIL - UNII:DR5S136IVO) AVANAFIL 50 mg Inactive Ingredients Ingredient Name Strength FUMARIC ACID (UNII: 88XHZ13131) HYDROXYPROPYL CELLULOSE (45000 WAMW) (UNII: 8VAB711C5E) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE (11% HYDROXYPROPYL; 130000 MW) (UNII: 7773C1ROEU) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) SODIUM BICARBONATE (UNII: 8MDF5V39QO) Product Characteristics Color white (white to off-white) Score no score Shape OVAL (biconvex) Size 7mm Flavor Imprint Code A41;H Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:31722-440-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/14/2024 2 NDC:31722-440-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 06/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209266 06/14/2024 AVANAFIL avanafil tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:31722-441 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVANAFIL (UNII: DR5S136IVO) (AVANAFIL - UNII:DR5S136IVO) AVANAFIL 100 mg Inactive Ingredients Ingredient Name Strength FUMARIC ACID (UNII: 88XHZ13131) HYDROXYPROPYL CELLULOSE (45000 WAMW) (UNII: 8VAB711C5E) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE (11% HYDROXYPROPYL; 130000 MW) (UNII: 7773C1ROEU) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) SODIUM BICARBONATE (UNII: 8MDF5V39QO) Product Characteristics Color white (white to off-white) Score no score Shape OVAL (biconvex) Size 9mm Flavor Imprint Code A42;H Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:31722-441-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/14/2024 2 NDC:31722-441-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 06/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209266 06/14/2024 AVANAFIL avanafil tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:31722-442 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVANAFIL (UNII: DR5S136IVO) (AVANAFIL - UNII:DR5S136IVO) AVANAFIL 200 mg Inactive Ingredients Ingredient Name Strength FUMARIC ACID (UNII: 88XHZ13131) HYDROXYPROPYL CELLULOSE (45000 WAMW) (UNII: 8VAB711C5E) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE (11% HYDROXYPROPYL; 130000 MW) (UNII: 7773C1ROEU) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) SODIUM BICARBONATE (UNII: 8MDF5V39QO) Product Characteristics Color white (white to off-white) Score no score Shape OVAL (biconvex) Size 12mm Flavor Imprint Code A43;H Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:31722-442-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/14/2024 2 NDC:31722-442-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 06/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209266 06/14/2024 Labeler - Camber Pharmaceuticals, Inc. (826774775)
CloseEstablishment Name Address ID/FEI Business Operations Hetero Labs Limited Unit III 676162024 manufacture(31722-440, 31722-441, 31722-442)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
AVANAFIL- avanafil tablet
Number of versions: 1
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Oct 21, 2024 | 1 (current) | download |
RxNorm
AVANAFIL- avanafil tablet
Get Label RSS Feed for this Drug
AVANAFIL- avanafil tablet
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=1d04e17d-7399-430a-a8bb-fa1912b155e9
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
AVANAFIL- avanafil tablet
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 31722-440-01 |
| 2 | 31722-440-30 |
| 3 | 31722-441-01 |
| 4 | 31722-441-30 |
| 5 | 31722-442-01 |
| 6 | 31722-442-30 |



