Label: ORPHENADRINE CITRATE tablet, extended release
- NDC Code(s): 67296-1077-1
- Packager: RedPharm Drug
- This is a repackaged label.
- Source NDC Code(s): 0185-0022
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only
-
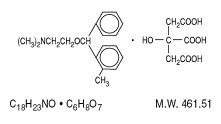
DESCRIPTIONOrphenadrine citrate, USP is the citrate salt of orphenadrine (2-dimethyl-aminoethyl 2-methylbenzhydryl ether citrate). It occurs as a white, crystalline powder having a bitter taste. It is ...
-
CLINICAL PHARMACOLOGYThe mode of therapeutic action has not been clearly identified, but may be related to its analgesic properties. Orphenadrine citrate does not directly relax tense skeletal muscles in man ...
-
INDICATIONS AND USAGEOrphenadrine citrate extended-release tablets are indicated as an adjunct to rest, physical therapy and other measures for the relief of discomfort associated with acute painful musculo skeletal ...
-
CONTRAINDICATIONSContraindicated in patients with glaucoma, pyloric or duodenal obstruction, stenosing peptic ulcers, prostatic hypertrophy or obstruction of the bladder neck, cardio-spasm (megaesophagus) and ...
-
WARNINGSSome patients may experience transient episodes of light-headedness, dizziness or syncope. Orphenadrine citrate may impair the ability of the patient to engage in potentially hazardous activities ...
-
PRECAUTIONSConfusion, anxiety and tremors have been reported in few patients receiving propoxyphene and orphenadrine concomitantly. As these symptoms may be simply due to an additive effect, reduction of ...
-
PREGNANCYAnimal reproduction studies have not been conducted with orphenadrine citrate. It is also not known whether orphenadrine citrate can cause fetal harm when administered to a pregnant woman or can ...
-
PEDIATRIC USESafety and effectiveness in pediatric patients have not been established.
-
ADVERSE REACTIONSAdverse reactions of orphenadrine citrate are mainly due to the mild anti-cholinergic action of orphenadrine citrate and are usually associated with higher dosage. Dryness of the mouth is usually ...
-
DRUG ABUSE AND DEPENDENCEOrphenadrine citrate has been chronically abused for its euphoric effects. [1]The mood elevating effects may occur at therapeutic doses of orphenadrine. [2]
-
OVERDOSAGEOrphenadrine citrate is toxic when overdosed and typically induces anticholinergic effects. [3]In a review of orphenadrine toxicity, the minimum lethal dose was found to be 2 to 3 grams for ...
-
DOSAGE AND ADMINISTRATIONAdults-Two tablets per day; one in the morning and one in the evening.
-
HOW SUPPLIEDOrphenadrine Citrate Extended-Release Tablets, USP, for oral administration, are available as - 100 mg - White, round-shaped tablets debossed “ E” over “22” on one side and plain on the other ...
-
Orphenadrine Citrate Extended-Release Tablets, USP, 100 mg x 100 Tablets - LabelNDC 0185-0022-01 - Orphenadrine Citrate Extended-Release Tablets, USP - 100 mg - Rx only - 100 Tablets - Sandoz
-
INGREDIENTS AND APPEARANCEProduct Information