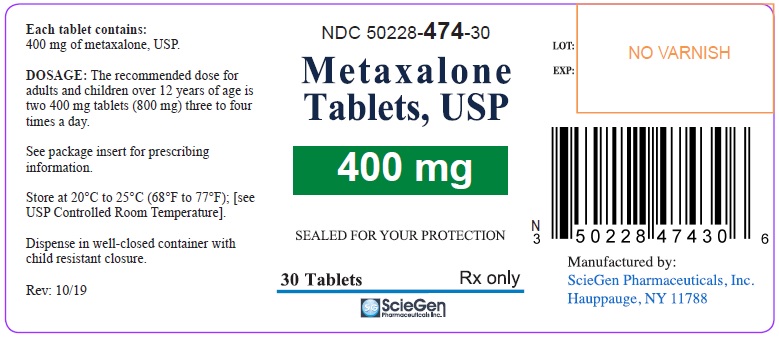
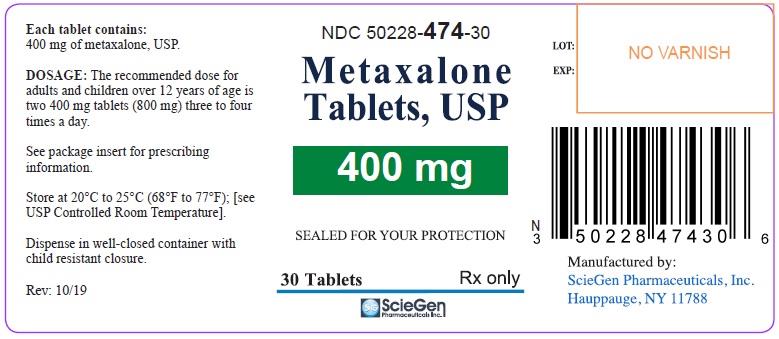
Label: METAXALONE tablet
- NDC Code(s): 50228-323-01, 50228-323-05, 50228-323-30, 50228-474-10, view more
- Packager: ScieGen Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use METAXALONE TABLETS safely and effectively. See full prescribing information for METAXALONE TABLETS. METAXALONE tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMetaxalone tablets are indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions in adults and ...
-
2 DOSAGE AND ADMINISTRATIONThe recommended dosage of metaxalone tablets in adults and pediatric patients 13 years of age and older is two 400 mg tablets (800 mg) or one 800 mg tablet orally three to four times a day [see ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 400 mg: round, light pink debossed with ‘SG 474’ on one side and plain on the other. 800 mg: capsule shape scored, pink debossed with ‘SG’ on scored side and ‘323’ on the other.
-
4 CONTRAINDICATIONSMetaxalone tablets are contraindicated in patients with: Known hypersensitivity to any component of metaxalone tablets. Known tendency to drug induced, hemolytic, or other anemias. Severe renal ...
-
5 WARNINGS AND PRECAUTIONS5.1 Serotonin Syndrome - Cases of serotonin syndrome, a potentially life-threatening condition, have been reported during concomitant use of metaxalone tablets (within the recommended dosage ...
-
6 ADVERSE REACTIONSThe following adverse reactions associated with the use of metaxalone tablets were identified in clinical studies or postmarketing reports. Because some of these reactions were reported ...
-
7 DRUG INTERACTIONS7.1 Serotonergic Drugs - If concomitant use of metaxalone tablets and another serotoneric drug is warranted, carefully observe the patient, particularly during treatment initiation and dosage ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on metaxalone use in pregnant patients to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse ...
-
10 OVERDOSAGEClinical Presentation of Metaxalone Overdose - Deaths by deliberate or accidental overdose have occurred with metaxalone, particularly in combination with other CNS depressants (including ...
-
11 DESCRIPTIONMetaxalone tablets , USP contain 400 mg and 800 mg of metaxalone and the following inactive ingredients: alginic acid, corn starch, ferric oxide red, copovidone, magnesium stearate, povidone ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Metaxalone’s mechanism of action has not been fully characterized, but may be related to its sedative properties. Metaxalone has no direct action on the contractile ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies to evaluate the carcinogenic potential of metaxalone have not been conducted. Studies to evaluate the mutagenic ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGMetaxalone Tablets, USP 400 mg are available as light pink, round shaped tablet debossed with ‘SG 474’ on one side and plain on the other. NDC 50228-474-30: Bottles of 30 Tablets - NDC 50228-474-10 ...
-
17 PATIENT COUNSELING INFORMATIONSerotonin Syndrome - Inform patients that metaxalone tablets could cause a rare but potentially life-threatening - condition called serotonin syndrome. Warn patients of the ...
-
PRINCIPAL DISPLAY PANELNDC 50228-474-30 - Metaxalone - Tablets, USP - 400 mg - SEALED FOR YOUR PROTECTION - 30 Tablets Rx only - ScieGen Pharmaceuticals, Inc. NDC 50228-474-10 - Metaxalone - Tablets ...
-
INGREDIENTS AND APPEARANCEProduct Information