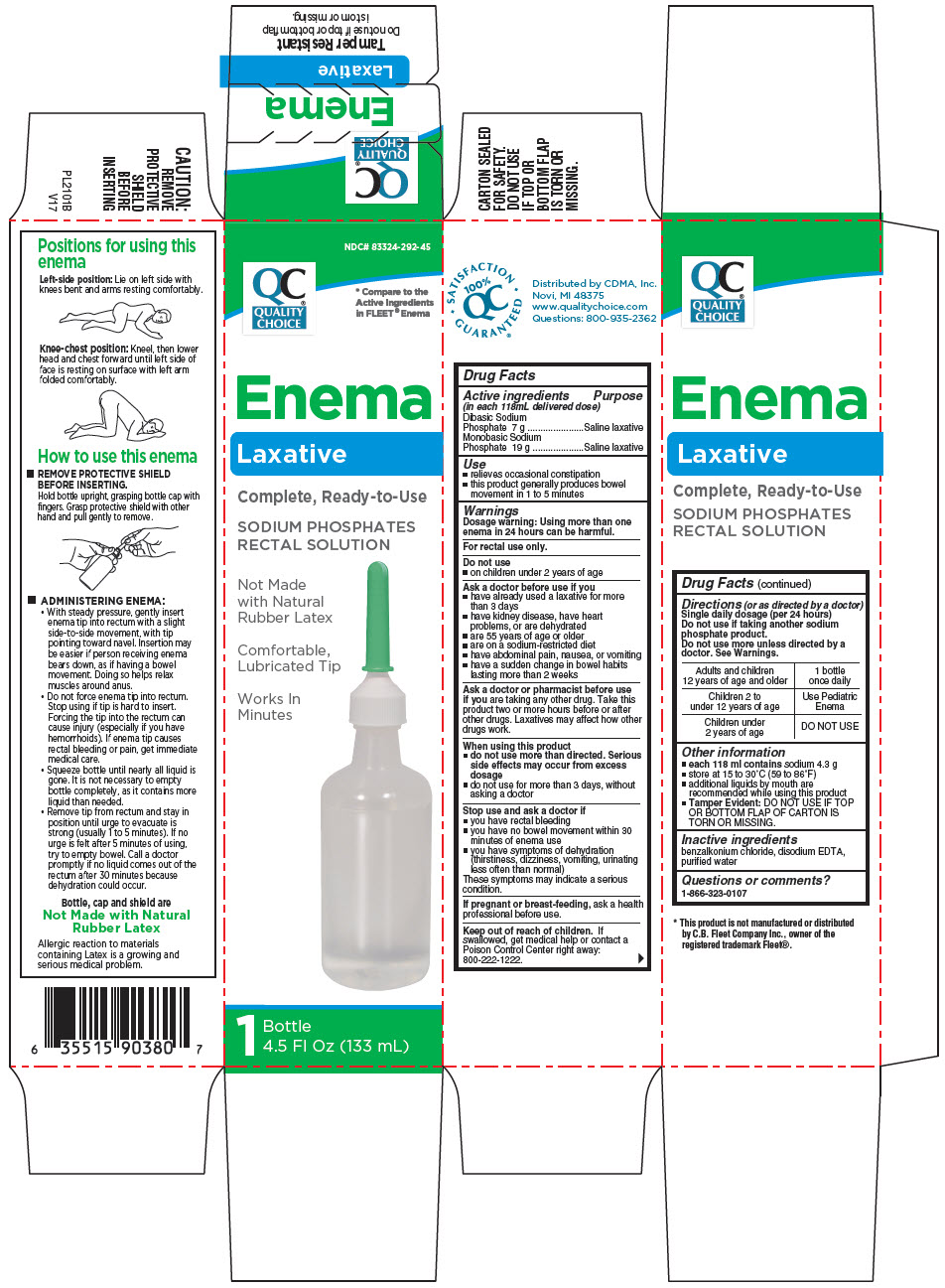
Label: QUALITY CHOICE LAXATIVE- sodium phosphate, dibasic, unspecified form and sodium phosphate, monobasic, unspecified form enema
- NDC Code(s): 83324-292-45, 83324-293-90
- Packager: Chain Drug Marketing Association
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Use
-
Warnings
Ask a doctor before use if you
- have already used a laxative for more than 3 days
- have kidney disease, have heart problems, or are dehydrated
- are 55 years of age or older
- are on a sodium-restricted diet
- have abdominal pain, nausea, or vomiting
- have a sudden change in bowel habits lasting more than 2 weeks
Ask a doctor or pharmacist before use if you are taking any other drug. Take this product two or more hours before or after other drugs. Laxatives may affect how other drugs work.
When using this product
- do not use more than directed. Serious side effects may occur from excess dosage
- do not use for more than 3 days, without asking a doctor
-
Directions (or as directed by a doctor)
Single daily dosage (per 24 hours)
Do not use if taking another sodium phosphate product.
Do not use more unless directed by a doctor. See Warnings.
Adults and children 12 years of age and older 1 bottle once daily Children 2 to under 12 years of age Use Pediatric Enema Children under 2 years of age DO NOT USE - Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 133 mL Bottle Carton - 1 Bottle
- PRINCIPAL DISPLAY PANEL - 133 mL Bottle Carton - 2 Bottles
-
INGREDIENTS AND APPEARANCE
QUALITY CHOICE LAXATIVE
sodium phosphate, dibasic, unspecified form and sodium phosphate, monobasic, unspecified form enemaProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83324-292 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) (PHOSPHATE ION - UNII:NK08V8K8HR) SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM 7 g in 118 mL SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) (PHOSPHATE ION - UNII:NK08V8K8HR) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM 19 g in 118 mL Inactive Ingredients Ingredient Name Strength Benzalkonium Chloride (UNII: F5UM2KM3W7) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83324-292-45 1 in 1 CARTON 06/01/2010 1 133 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M007 06/01/2010 QUALITY CHOICE LAXATIVE
sodium phosphate, dibasic, unspecified form and sodium phosphate, monobasic, unspecified form enemaProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83324-293 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) (PHOSPHATE ION - UNII:NK08V8K8HR) SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM 7 g in 118 mL SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) (PHOSPHATE ION - UNII:NK08V8K8HR) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM 19 g in 118 mL Inactive Ingredients Ingredient Name Strength Benzalkonium Chloride (UNII: F5UM2KM3W7) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83324-293-90 2 in 1 CARTON 06/01/2010 1 133 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M007 06/01/2010 Labeler - Chain Drug Marketing Association (011920774) Establishment Name Address ID/FEI Business Operations Natureplex LLC 062808196 MANUFACTURE(83324-292, 83324-293)