Label: BUTALBITAL, ACETAMINOPHEN AND CAFFEINE tablet
- NDC Code(s): 63187-520-30, 63187-520-60, 63187-520-90
- Packager: Proficient Rx LP
- This is a repackaged label.
- Source NDC Code(s): 46672-053
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIII
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 1, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
WARNINGHEPATOTOXICITY - ACETAMINOPHEN HAS BEEN ASSOCIATED WITH CASES OF ACUTE LIVER FAILURE, AT TIMES RESULTING IN LIVER TRANSPLANT AND DEATH. MOST OF THE CASES OF LIVER INJURY ARE ASSOCIATED WITH ...
-
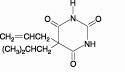
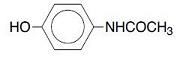
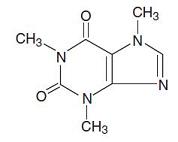
DESCRIPTIONButalbital, acetaminophen and caffeine are supplied in tablet form for oral administration. Each tablet contains the following active ingredients: Butalbital ...................50 mg - Warning: May ...
-
CLINICAL PHARMACOLOGYThis combination drug product is intended as a treatment for tension headache. It consists of a fixed combination of butalbital, acetaminophen and caffeine. The role each component plays in the ...
-
INDICATIONS AND USAGEButalbital, acetaminophen and caffeine tablets are indicated for the relief of the symptom complex of tension (or muscle contraction) headache. Evidence supporting the efficacy and safety of this ...
-
CONTRAINDICATIONSThis product is contraindicated under the following conditions: • Hypersensitivity or intolerance to any component of this product. • Patients with porphyria.
-
WARNINGSButalbital is habit-forming and potentially abusable. Consequently, the extended use of this product is not recommended. Hepatotoxicity - Acetaminophen has been associated with cases of acute ...
-
PRECAUTIONSGeneral - Butalbital, acetaminophen and caffeine tablets should be prescribed with caution in certain special-risk patients, such as the elderly or debilitated, and those with severe impairment ...
-
ADVERSE REACTIONSFrequently Observed - The most frequently reported adverse reactions are drowsiness, lightheadedness, dizziness, sedation, shortness of breath, nausea, vomiting, abdominal pain, and intoxicated ...
-
DRUG ABUSE AND DEPENDENCE:Abuse and Dependence - Butalbital: Barbiturates may be habit-forming: Tolerance, psychological dependence, and physical dependence may occur especially following prolonged use of high doses of ...
-
OVERDOSAGEFollowing an acute overdosage of butalbital, acetaminophen and caffeine, toxicity may result from the barbiturate or the acetaminophen. Toxicity due to caffeine is less likely, due to the ...
-
DOSAGE AND ADMINISTRATIONOne or two tablets every four hours as needed. Total daily dosage should not exceed 6 tablets. Extended and repeated use of this product is not recommended because of the potential for physical ...
-
HOW SUPPLIEDButalbital, acetaminophen and caffeine tablets USP contain butalbital 50 mg (Warning: May be habit-forming), acetaminophen 325 mg and caffeine 40 mg. Tablets are white, capsule-shaped ...
-
SPL UNCLASSIFIED SECTIONRx only - Manufactured by: MIKART, INC. Atlanta, GA 30318 - Code 687Z00 - Rev. 10/13 - Repackaged By: Proficient Rx LP - Thousand Oaks, CA 91320
-
PRINCIPAL DISPLAY PANEL

-
INGREDIENTS AND APPEARANCEProduct Information