Label: GENTAMICIN SULFATE solution/ drops
- NDC Code(s): 67296-0370-1
- Packager: Redpharm Drug
- This is a repackaged label.
- Source NDC Code(s): 60758-188
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
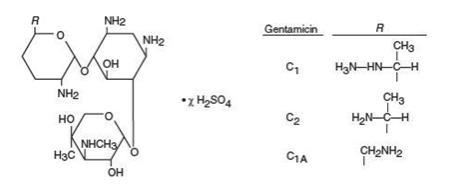
Gentamicin sulfate ophthalmic solution, USP is a sterile, topical anti-infective agent for ophthalmic use. Gentamicin is obtained from cultures of - Micromonospora purpurea. It is a mixture of ...
-
CLINICAL PHARMACOLOGY
Microbiology - Gentamicin sulfate is active - in vitroagainst many strains of the following microorganisms: Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes ...
-
INDICATIONS AND USAGE
Gentamicin sulfate ophthalmic solution, USP is indicated in the topical treatment of ocular bacterial infections including conjunctivitis, keratitis, keratoconjunctivitis, corneal ulcers ...
-
CONTRAINDICATIONS
Gentamicin sulfate ophthalmic solution, USP is contraindicated in patients with known hypersensitivity to any of its components.
-
WARNINGS
NOT FOR INJECTION INTO THE EYE. Gentamicin sulfate ophthalmic solution, USP is not for injection. It should never be injected subconjunctivally, nor should it be directly introduced into the ...
-
PRECAUTIONS
General - Prolonged use of topical antibiotics may give rise to overgrowth of nonsusceptible microorganisms, including fungi. Bacterial resistance to gentamicin may also develop. If purulent ...
-
ADVERSE REACTIONS
Bacterial and fungal corneal ulcers have developed during treatment with gentamicin ophthalmic preparations. The most frequently reported adverse reactions are ocular burning and irritation upon ...
-
DOSAGE AND ADMINISTRATION
Instill one or two drops into the affected eye(s) every four hours. In severe infections, dosage may be increased to as much as two drops every hour.
-
HOW SUPPLIED
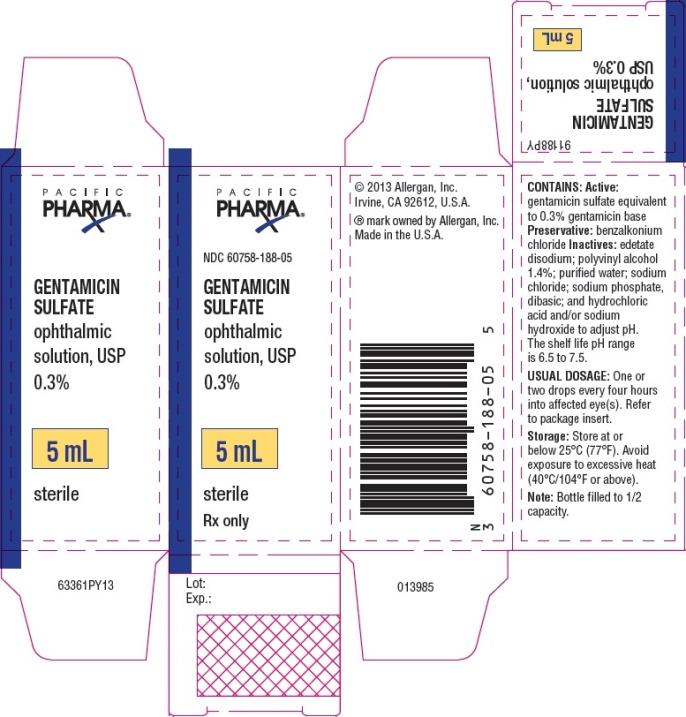
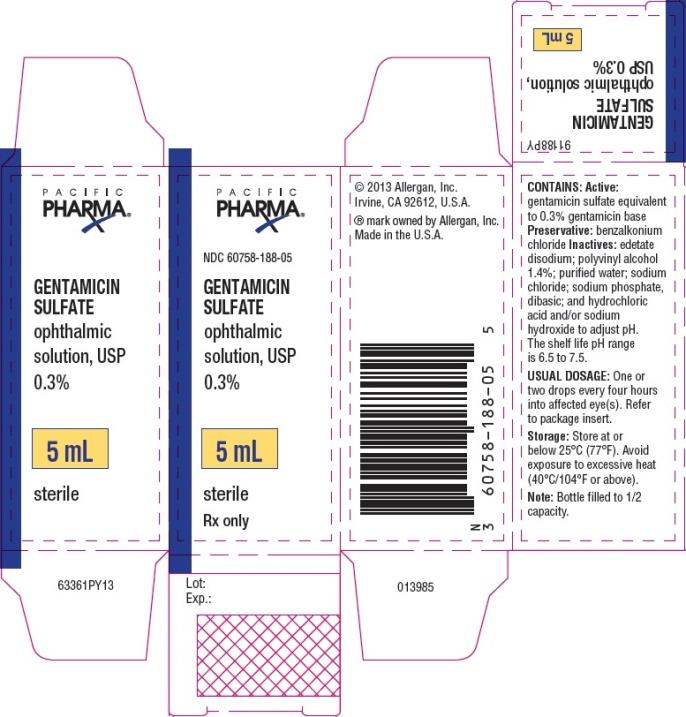
Gentamicin sulfate ophthalmic solution, USP 0.3% is supplied sterile in white opaque LDPE plastic bottles and tips with beige high impact polystyrene (HIPS) caps as follows: 5 mL in 10 mL bottle ...
-
PRINCIPAL DISPLAY PANEL
NDC 60758-188-05 - GENTAMICIN - SULFATE - ophthalmic - solution, USP - 0.3% 5 mL - sterile - Rx Only
-
INGREDIENTS AND APPEARANCEProduct Information