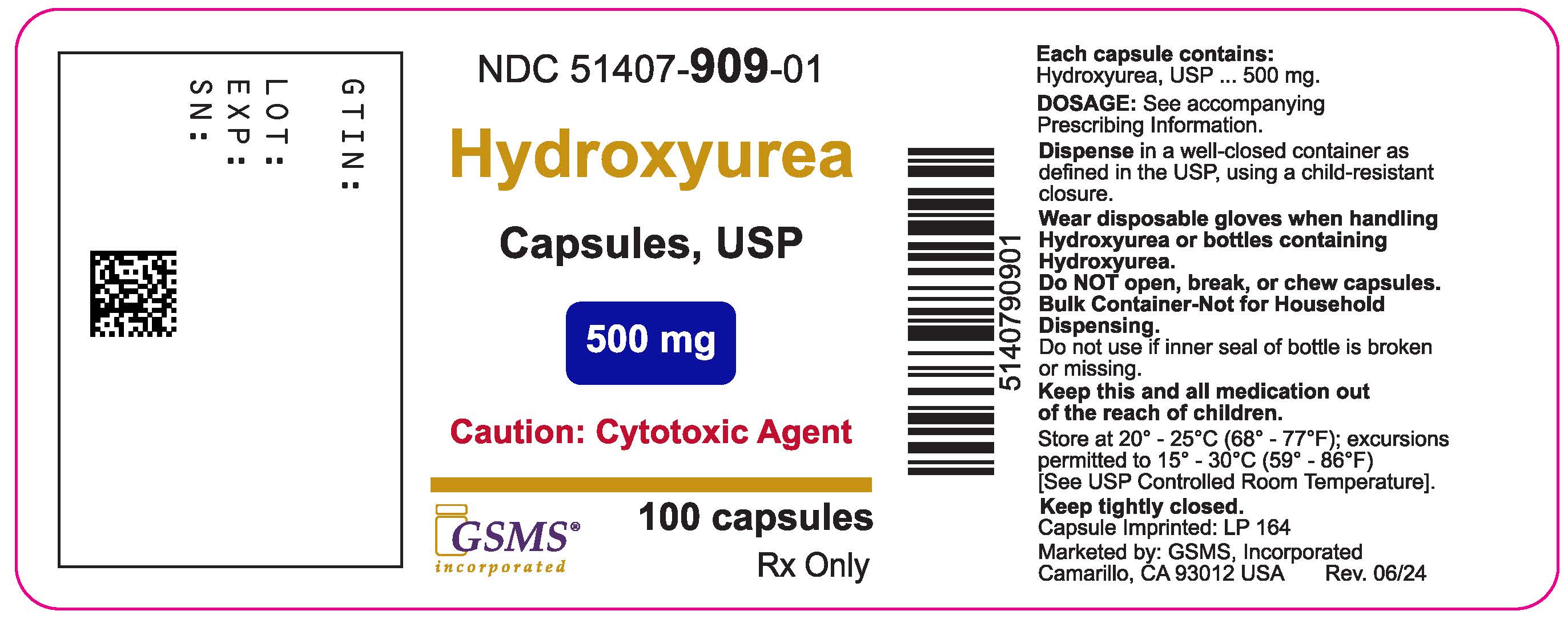
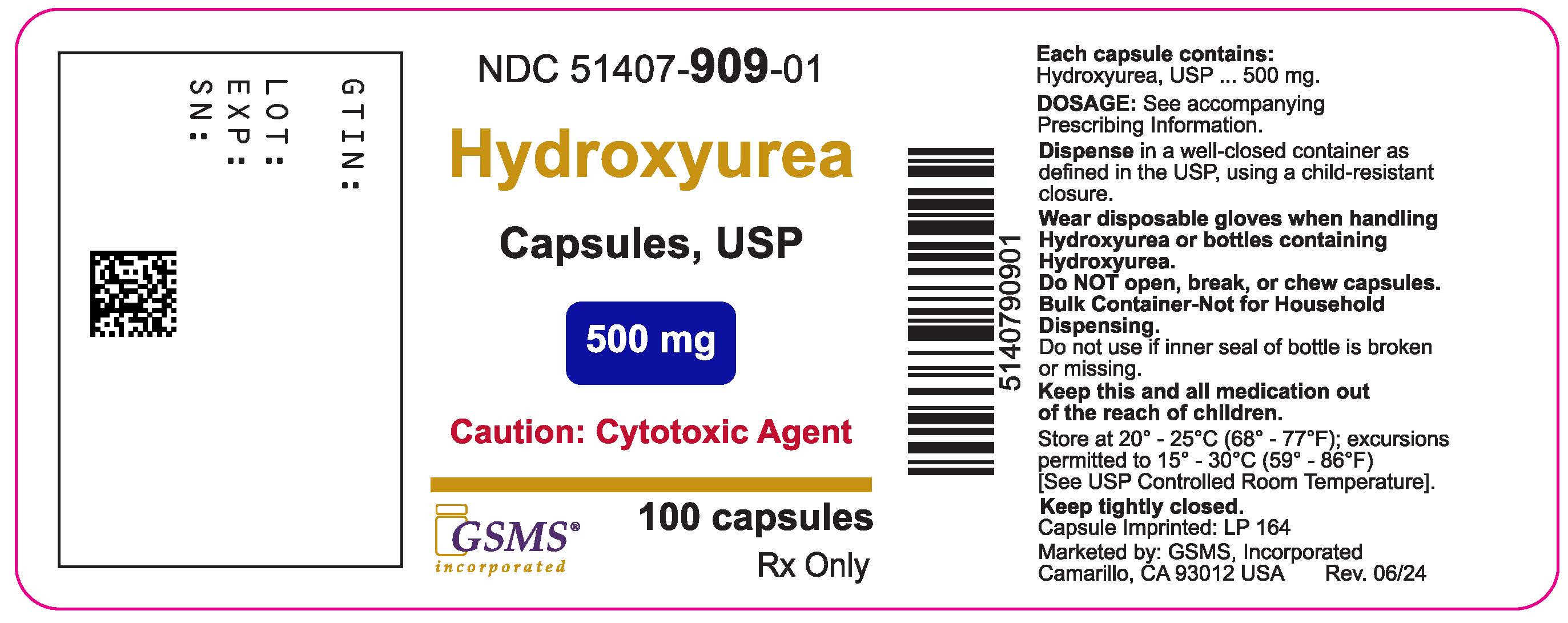
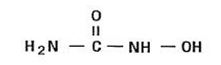Label: HYDROXYUREA capsule
- NDC Code(s): 51407-909-01
- Packager: Golden State Medical Supply, Inc.
- This is a repackaged label.
- Source NDC Code(s): 69315-164
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONHYDROXYUREA CAPSULES. These highlights do not include all the information needed to use HYDROXYUREA CAPSULES safely and effectively. See full prescribing information for HYDROXYUREA CAPSULES ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEHydroxyurea capsules, USP is indicated for the treatment of: Resistant chronic myeloid leukemia. Locally advanced squamous cell carcinomas of the head and neck (excluding the lip) in combination ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - Hydroxyurea is used alone or in conjunction with other antitumor agents or radiation therapy to treat neoplastic diseases. Individualize treatment based on tumor type ...
-
3 DOSAGE FORMS AND STRENGTHSCapsules: 500 mg, green opaque cap imprinted in black with “LP 164” and light pink opaque body imprinted in black with “LP 164”
-
4 CONTRAINDICATIONSHydroxyurea capsules is contraindicated in patients who have demonstrated a previous hypersensitivity to hydroxyurea or any other component of the formulation.
-
5 WARNINGS AND PRECAUTIONS5.1 Myelosuppression s ion - Hydroxyurea causes severe myelosuppression. Treatment with hydroxyurea should not be initiated if bone marrow function is markedly depressed. Bone marrow suppression ...
-
6 ADVERSE REACTIONS• Myelosuppression - [see - Warnings and Precautions (5.1)] • Hemolytic anemia - [see - Warnings and Precautions (5.2)] • Malignancies - [see - Warnings and ...
-
7 DRUG INTERACTIONS7.1 Increased Toxicity with Concomitant Use of Antiretroviral Drugs - Pancreatitis - In patients with HIV infection during therapy with hydroxyurea and didanosine, with or without stavudine, fatal ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Hydroxyurea capsules can cause fetal harm based on findings from animal studies and the drug’s mechanism of action - [see - Clinical Pharmacology (12.1)] ...
-
10 OVERDOSAGEAcute mucocutaneous toxicity has been reported in patients receiving hydroxyurea at dosages several times the therapeutic dose. Soreness, violet erythema, edema on palms and soles followed by ...
-
11 DESCRIPTIONHydroxyurea Capsules USP is an antimetabolite available for oral use as capsules containing 500 mg hydroxyurea, USP. Inactive ingredients include Colorants (D&C Yellow No. 10, FD&C Red No.3, FD&C ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The precise mechanism by which hydroxyurea produces its antineoplastic effects cannot, at present, be described. However, the reports of various studies in tissue ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Conventional long-term studies to evaluate the carcinogenic potential of hydroxyurea capsules have not been performed. However ...
-
15 REFERENCESOSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html.
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Hydroxyurea capsules, USP is supplied as 500 mg capsules in HDPE bottles with heat induction Child Resistant Closures. Each bottle contains 100 capsules. The cap is opaque ...
-
17 PATIENT COUNSELING INFORMATIONThere is a risk of myelosuppression. Monitoring blood counts weekly throughout the duration of therapy should be emphasized to patients taking hydroxyurea capsules - . Advise patients to report ...
-
Hydroxyurea capsules, USP container label
-
INGREDIENTS AND APPEARANCEProduct Information