Label: PIRFENIDONE tablet
- NDC Code(s): 51407-913-27, 51407-914-90
- Packager: Golden State Medical Supply, Inc.
- This is a repackaged label.
- Source NDC Code(s): 60505-4681, 60505-4682
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONPIRFENIDONE TABLETS. These highlights do not include all the information needed to use PIRFENIDONE TABLETS safely and effectively. See full prescribing information for PIRFENIDONE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEPirfenidone tablets are indicated for the treatment of idiopathic pulmonary fibrosis (IPF).
-
2 DOSAGE AND ADMINISTRATION2.1 Testing Prior to Pirfenidone Tablets Administration - Conduct liver function tests prior to initiating treatment with pirfenidone tablets - [see Warnings and Precautions ( 5.1)] ...
-
3 DOSAGE FORMS AND STRENGTHS267 mg tablets: yellow, oval, biconvex, film-coated tablets. Engraved "APO" on one side, "267" on the other side. 801 mg tablets: brown, oval, biconvex, film-coated tablet. Engraved "APO" on one ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Elevated Liver Enzymes and Drug-Induced Liver Injury - Cases of drug-induced liver injury (DILI) have been observed with pirfenidone. In the postmarketing period, non-serious and serious ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of the labeling: Liver Enzyme Elevations and Drug-Induced Liver Injury - [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 CYP1A2 Inhibitors - Pirfenidone is metabolized primarily (70 to 80%) via CYP1A2 with minor contributions from other CYP isoenzymes including CYP2C9, 2C19, 2D6 and 2E1. Strong CYP1A2 ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The data with pirfenidone use in pregnant women are insufficient to inform on drug associated risks for major birth defects and miscarriage. In animal reproduction ...
-
10 OVERDOSAGEThere is limited clinical experience with overdosage. Multiple dosages of pirfenidone up to a maximum tolerated dose of 4005 mg per day were administered as five 267 mg capsules three times daily ...
-
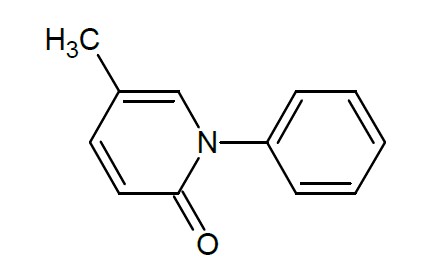
11 DESCRIPTIONPirfenidone belongs to the chemical class of pyridone. Pirfenidone tablets are available as film-coated tablets containing 267 mg (yellow) and 801 mg (brown) pirfenidone. Pirfenidone has a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of pirfenidone in the treatment of IPF has not been established. 12.2 Pharmacodynamics - Cardiac Electrophysiology: The effect of ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term studies were conducted in mice and rats with admixture of pirfenidone to the diet to evaluate its ...
-
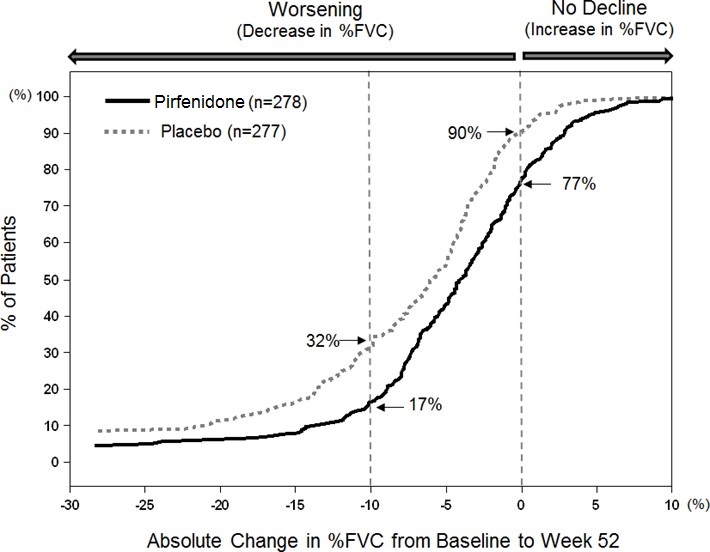
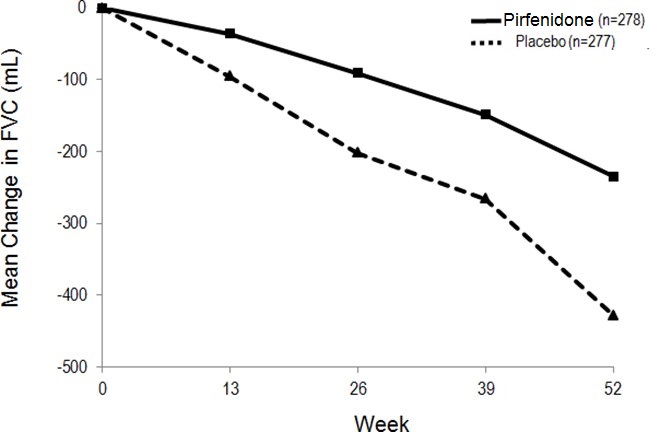
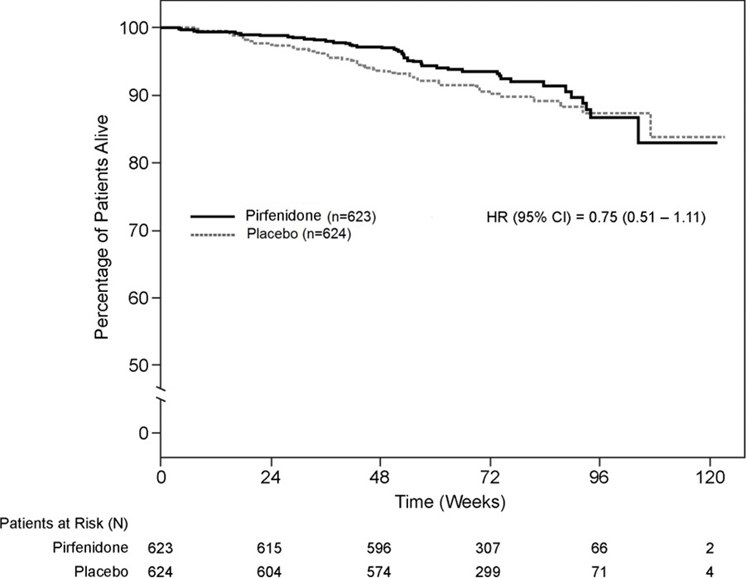
14 CLINICAL STUDIESThe efficacy of pirfenidone was evaluated in patients with IPF in three phase 3, randomized, double-blind, placebo-controlled, multicenter trials (Studies 1, 2, and 3). Study 1 was a 52-week trial ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGPirfenidone film-coated tablets contain 267 mg pirfenidone (yellow) and 801 mg pirfenidone (brown) and are available as follows: 267 mg tablets: Yellow, oval, biconvex, film-coated tablets ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Liver Enzyme Elevations - Advise patients that they may be required to undergo liver function testing ...
-
Patient InformationPirfenidone - Tablets, USP - (pir fen’ i done) What are pirfenidone tablets? Pirfenidone tablets are a prescription medicine used to treat people with a lung disease called idiopathic ...
-
PRINCIPAL DISPLAY PANELRepresentative sample of labeling (see HOW SUPPLIED section of complete listing): NDC 51407-913-27 - 267 mg - Rx only
-
PRINCIPAL DISPLAY PANELRepresentative sample of labeling (see HOW SUPPLIED section of complete listing): NDC 51407-914-90 - 801 mg - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information