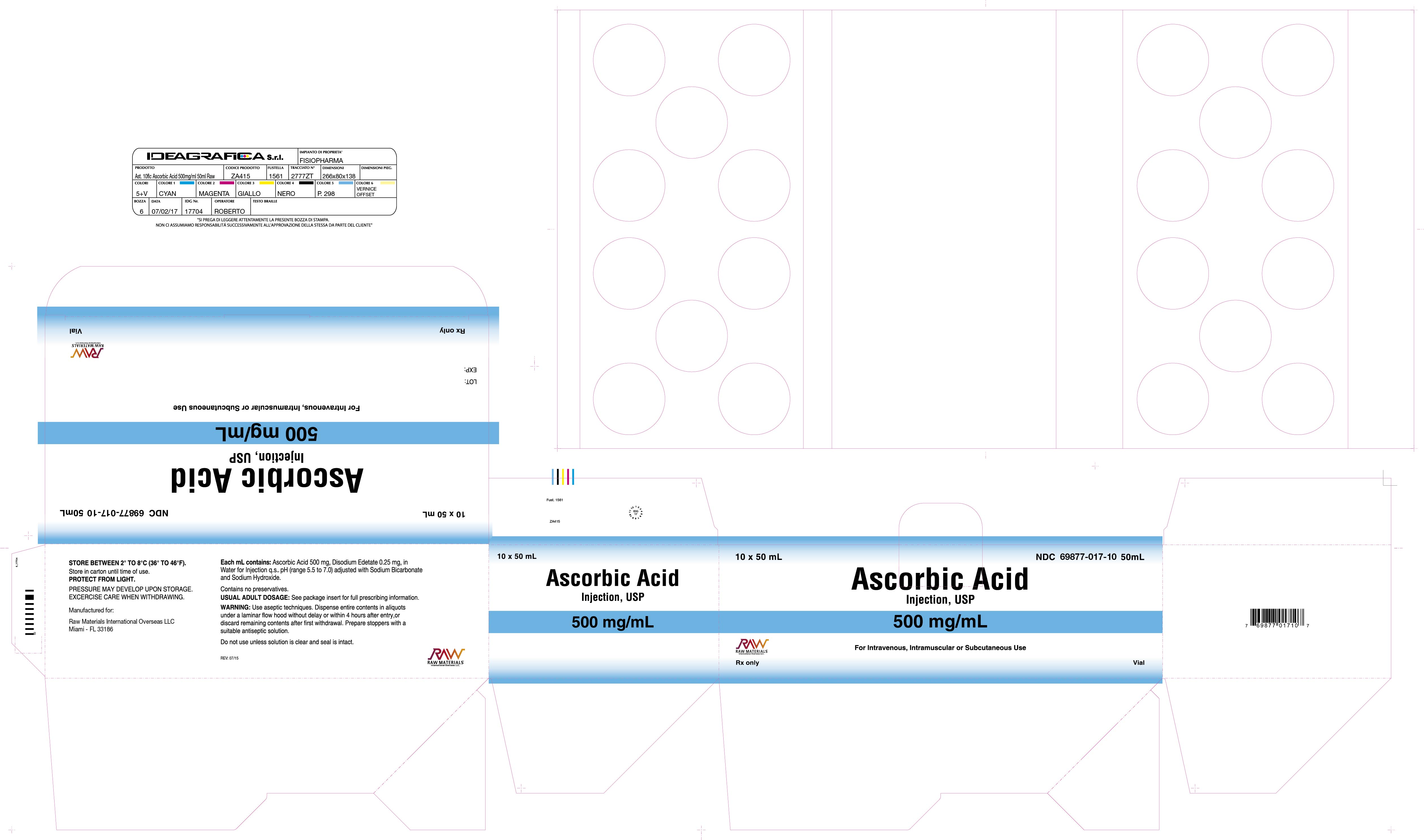
Label: ASCORBIC ACID injection, solution
- NDC Code(s): 69877-017-01
- Packager: Raw Materials International Overseas LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Ascorbic Acid (vitamin C) is a water-soluble vitamin. It occurs as a white or slightly yellow crystal or powder with a light acidic taste. It is an antiscorbutic product. On exposure to air and light it gradually darkens. In the dry state it is reasonably stable in air, but in solution it rapidly oxidizes. Ascorbic Acid is freely soluble in water; sparingly soluble in alcohol; insoluble in chloroform, ether, and benzene.
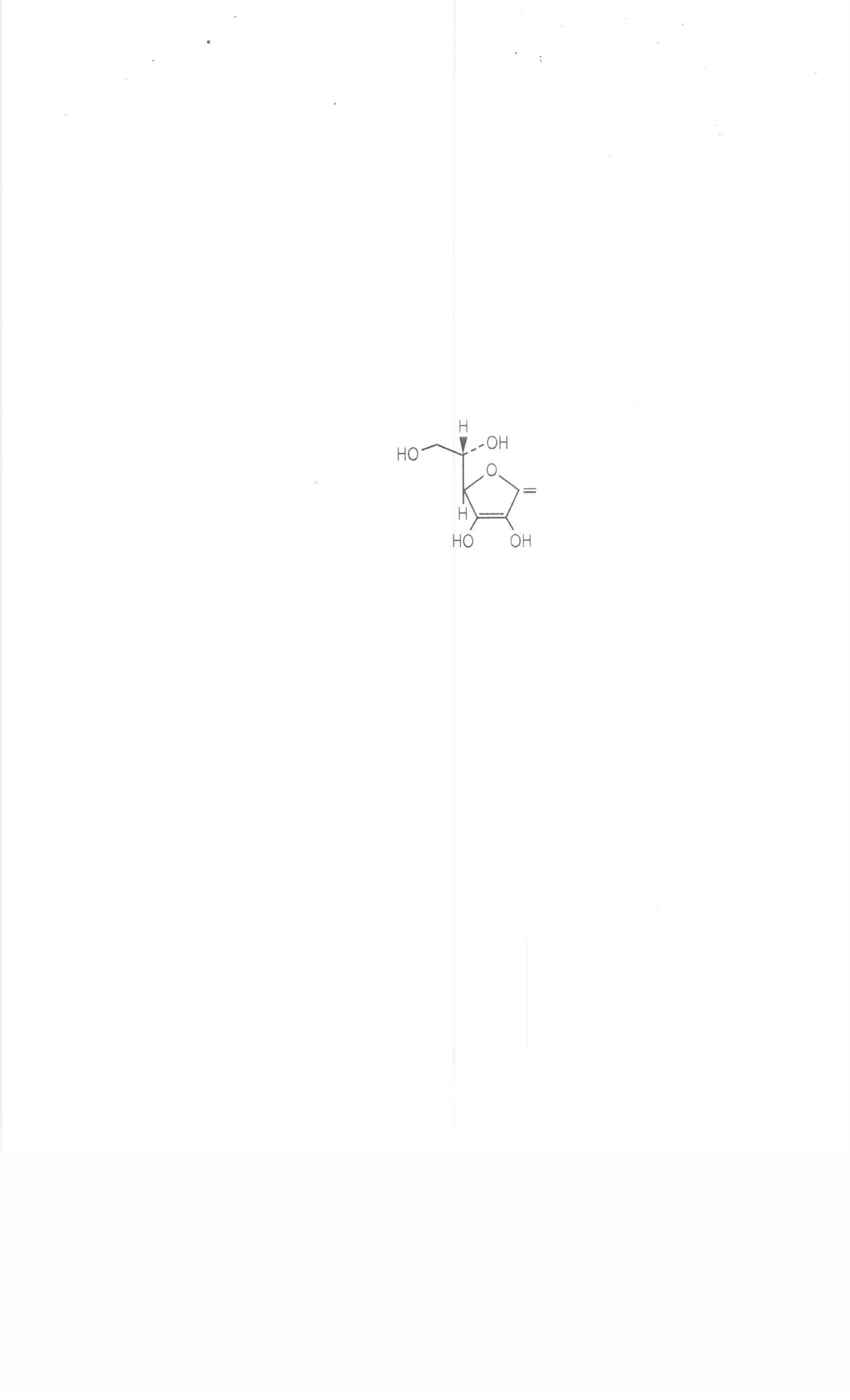
The chemical name of Ascorbic Acid is L-ascorbic acid. The molecular formula is C 6H 80 6 and the molecular weight is 176.13. The structural formula is as follows:
Ascorbic Acid injection is a clear, colorless to slightly yellow sterile solution of Ascorbic Acid in Water for Injection, for intravenous, intramuscular or subcutaneous use. Each mL contains: Ascorbic Acid 500 mg, Edetate Disodium 0.25 mg, Water for Injection q.s. pH (range 5.5-7.0) adjusted with Sodium Bicarbonate and Sodium Hydroxide. Contains no preservatives.
-
CLINICAL PHARMACOLOGY
In humans, an exogenous source of ascorbic acid is required for collagen formulation and tissue repair. Ascorbic acid is reversibly oxidized to dehydroascorbic acid in the body. These two forms of the vitamin are believed to be important in oxidation-reduction reactions. The vitamin is involved in tyrosine metabolism, conversion of folic acid to folinic acid, carbohydrate metabolism, synthesis of lipids and proteins, iron metabolism, resistance to infections, and cellular respiration.
Ascorbic acid deficiency results in scurvy. Collagenous structures are primarily affected, lesions develop in bones and blood vessels. Administration of ascorbic acid completely reverses the symptoms of ascorbic acid deficiency.
-
INDICATIONS & USAGE
Ascorbic acid is recommended for the prevention and treatment of scurvy. Its parenteral administration is desirable for patients with an acute deficiency or for those whose absorption of orally ingested ascorbic acid is uncertain.
Symptoms of mild deficiency may include faulty bone and tooth development, gingivitis, bleeding gums, and loosened teeth. Febrile states, chronic illness, and infection (pneumonia, whooping cough, tuberculosis, diphtheria, sinusitis, rheumatic fever, etc.) increases the need for ascorbic acid. Hemovascular disorders, burns, delayed fracture and wound healing are indications for an increase in the daily intake.
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
General Precautions: Too-rapid intravenous injection is to be avoided.
Laboratory Tests: Diabetics taking more than 500 mg of ascorbic acid daily, may obtain false reading of the urinary glucose test. No exogenous ascorbic acid should be ingested for 48 to 72 hours before amine dependent stool occult blood tests are conducted because possible false negative results may occur.
Drug Interactions: Limited evidence suggests that ascorbic acid may influence the intensity and duration of action of bishydroxycoumarin.
Usage in Pregnancy: Pregnancy Category C
Animal reproduction studies have not been conducted with Ascorbic Acid Injection. It is also not known whether Ascorbic Acid Injection can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Ascorbic Acid Injection should be given to a pregnant woman only if clearly needed.
Nursing Mothers: Caution should be exercised when Ascorbic Acid Injection is administered to a nursing woman.
- ADVERSE REACTIONS
-
DOSAGE & ADMINISTRATION
Ascorbic acid is usually administered orally. When oral administration is not feasible or when malabsorption is suspected, the drug may be administered intramuscularly, intravenously or subcutaneously. When given parenterally, utilization of the vitamin reportedly is best after IM administration, which is the preferred parenteral route.
For intravenous injection, dilution into a large volume parenteral such as Normal Saline or Glucose is recommended to minimize the adverse reactions associated with intravenous injection.
The average protective dose of ascorbic acid for adults is 70 to 150 mg daily. In the presence of scurvy, doses of 300 mg to 1 gram daily are recommended. However, as much as 6 grams have been administered parenterally to normal adults without evidence of toxicity.
To enhance wound-healing, doses of 300 to 500 mg daily for a week to ten days, both preoperatively and postoperatively, are generally considered adequate, although considerably larger amounts have been recommended. In the treatment of burns, doses are governed by the extent of tissue injury. For severe burns, daily doses of 1 to 2 grams are recommended. In other conditions in which the need for ascorbic acid is increased, three to five times the daily optimum allowances appear to be adequate.
-
WARNINGS
PRESSURE MAY DEVELOP WITHIN THE VIAL UPON STORAGE.
Exercise care when withdrawing and/or relieve pressure by first inserting sterile empty syringe into vial thus allowing pressure to equilibrate.
When using dispensing vials use aseptic technique. Dispense entire contents in aliquots under a laminar flow hood without delay or within 4 hours after entry or discard remaining content after first withdrawal. Prepare stoppers with a suitable antiseptic solution. Do not use unless solution is clear and seal is intact.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever the solution and container permit.
-
HOW SUPPLIED
Ascorbic Acid for Injection, USP (500mg/mL)
NDC 69877-017-01
50mL Sterile Dispensing Vial, individually boxed, Rx only
NDC 69877-017-10
50 mL Sterile Dinspensing Vial, packaged in boxes of 10 vials, Rx only
PROTECT FROM LIGHT. STORE IN CARTON UNTIL TIMEOF USE: Store between 2° to 8°C (36° to 46°F).
Rev. 07/15
- SPL UNCLASSIFIED SECTION
- Ascorbic acid - Box 1 vial Ascorbic acid - Box 10 vials Ascorbic acid leaflet
-
INGREDIENTS AND APPEARANCE
ASCORBIC ACID
ascorbic acid injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69877-017 Route of Administration SUBCUTANEOUS, INTRAVENOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 500 mg in 1 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) 0.25 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) SODIUM BICARBONATE (UNII: 8MDF5V39QO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69877-017-01 1 in 1 BOX 07/02/2015 12/31/2016 1 50 mL in 1 VIAL, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/02/2015 12/31/2016 Labeler - Raw Materials International Overseas LLC (079829477) Registrant - Fisiopharma Srl (441067444) Establishment Name Address ID/FEI Business Operations Fisiopharma Srl 441067444 manufacture(69877-017)