Label: PIRFENIDONE- pirfenidone tablet, film coated
- NDC Code(s): 42571-335-39, 42571-335-90, 42571-336-90
- Packager: Micro Labs Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 4, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PIRFENIDONE TABLETS safely and effectively. See full prescribing information for PIRFENIDONE TABLETS. PIRFENIDONE tablets, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEPirfenidone tablet is indicated for the treatment of idiopathic pulmonary fibrosis (IPF).
-
2 DOSAGE AND ADMINISTRATION2.1 Testing Prior to Pirfenidone Tablet Administration - Conduct liver function tests prior to initiating treatment with pirfenidone tablets - [see - Warnings and Precautions (5.1)] ...
-
3 DOSAGE FORMS AND STRENGTHSPirfenidone Tablets 267 mgare Yellow to pale yellow colored, oval shaped, biconvex film coated tablets plain on one side and debossed with 'P1' on the other side. Pirfenidone Tablets 801 ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Elevated Liver Enzymes and Drug-Induced Liver Injury - Cases of drug-induced liver injury (DILI) have been observed with pirfenidone tablets. In the postmarketing period, non-serious and ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of the labeling: Liver Enzyme Elevations and Drug-Induced Liver Injury - [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 CYP1A2 Inhibitors - Pirfenidone is metabolized primarily (70 to 80%) via CYP1A2 with minor contributions from other CYP isoenzymes including CYP2C9, 2C19, 2D6 and 2E1. Strong CYP1A2 ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The data with pirfenidone tablets use in pregnant women are insufficient to inform on drug associated risks for major birth defects and miscarriage. In animal ...
-
10 OVERDOSAGEThere is limited clinical experience with overdosage. Multiple dosages of pirfenidone tablets up to a maximum tolerated dose of 4005 mg per day were administered as five 267 mg capsules three ...
-
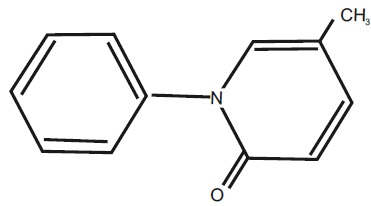
11 DESCRIPTIONPirfenidone tablets belong to the chemical class of pyridone. Pirfenidone tablets are available as film-coated tablets containing 267 mg (yellow) and 801 mg (brown) pirfenidone. Pirfenidone has ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of pirfenidone in the treatment of IPF has not been established. 12.2 Pharmacodynamics - Cardiac Electrophysiology: The effect of ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term studies were conducted in mice and rats with admixture of pirfenidone to the diet to evaluate its ...
-
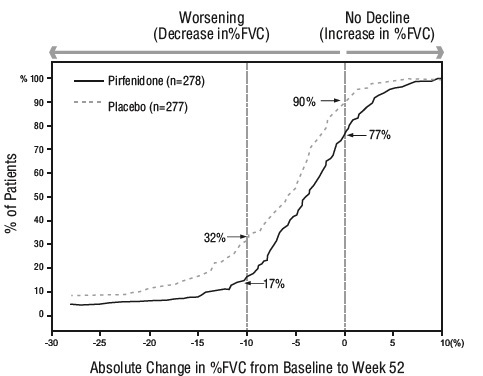
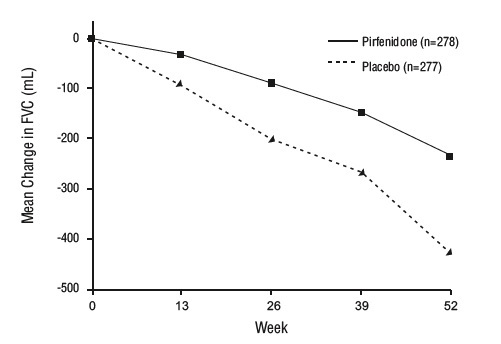
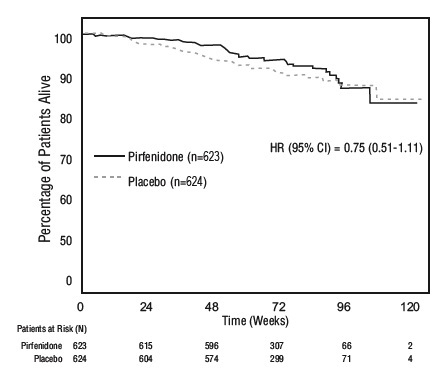
14 CLINICAL STUDIESThe efficacy of pirfenidone tablets was evaluated in patients with IPF in three phase 3, randomized, double-blind, placebo-controlled, multicenter trials (Studies 1, 2, and 3). Study 1 was a ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGPirfenidone Tablets 267 mg are Yellow to pale yellow colored, oval shaped, biconvex film coated tablets plain on one side and debossed with 'P1' on the other side. 90’s Count Bottle in a ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Liver Enzyme Elevations - Advise patients that they may be required to undergo liver function testing ...
-
Patient InformationPirfenidone (pir fen’ i done) Tablets - What are pirfenidone tablets? Pirfenidone tablets are a prescription medicine used to treat people with a lung disease called idiopathic pulmonary ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 42571-335-90 - Pirfenidone Tablets - 267 mg - Rx only - 90 Tablets - MICRO LABS - NDC 42571-335-39 - Pirfenidone Tablets 267 mg - Rx only 270 ...
-
INGREDIENTS AND APPEARANCEProduct Information