Label: CALCIUM GLUCONATE- calcium gluconate injection, solution
- NDC Code(s): 65219-630-01, 65219-630-19
- Packager: Fresenius Kabi USA, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CALCIUM GLUCONATE INJECTION safely and effectively. See full prescribing information for CALCIUM GLUCONATE INJECTION. CALCIUM ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
Calcium Gluconate Injection is indicated for pediatric and adult patients for the treatment of acute symptomatic hypocalcemia. Limitations of Use - The safety of Calcium Gluconate Injection ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions - Calcium Gluconate Injection contains 100 mg of calcium gluconate per mL which contains 9.3 mg (i.e., 0.465 mEq) of elemental calcium. Dilute Calcium ...
-
3 DOSAGE FORMS AND STRENGTHS
Calcium Gluconate Injection, USP is a clear, colorless to slightly yellow, solution available in the following: Single dose vial: 1,000 mg per 10 mL (100 mg per mL) Each mL of Calcium ...
-
4 CONTRAINDICATIONS
Calcium Gluconate Injection is contraindicated in: Hypercalcemia - Neonates (28 days of age or younger) receiving ceftriaxone [see Warnings and Precautions (5.2)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Arrhythmias with Concomitant Cardiac Glycoside Use - Cardiac arrhythmias may occur if calcium and cardiac glycosides are administered together. Hypercalcemia increases the risk of digoxin ...
-
6 ADVERSE REACTIONS
The following serious adverse reactions are also described elsewhere in the labeling: Arrhythmias with Concomitant Cardiac Glycoside Use [see Warnings and Precautions (5.1)] End-Organ Damage ...
-
7 DRUG INTERACTIONS
7.1 Cardiac Glycosides - Hypercalcemia increases the risk of digoxin toxicity, while digoxin may be therapeutically ineffective in the presence of hypocalcemia. Synergistic arrhythmias may occur ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk summary - Limited available data with Calcium Gluconate Injection use in pregnant women are insufficient to inform a drug associated risk of adverse developmental ...
-
10 OVERDOSAGE
Overdosage of Calcium Gluconate Injection may result in hypercalcemia. Symptoms of hypercalcemia typically develop when the total serum calcium concentration is ≥12 mg/dL. Neurologic symptoms ...
-
11 DESCRIPTION
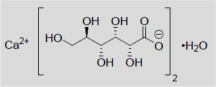
Calcium Gluconate Injection, USP is a sterile, preservative-free, nonpyrogenic, supersaturated solution of calcium gluconate, a form of calcium, for intravenous use. Calcium Gluconate is calcium ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Intravenous administration of calcium gluconate increases serum ionized calcium level. Calcium gluconate dissociates into ionized calcium in plasma. Ionized calcium ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals have not been conducted to evaluate the carcinogenic potential of Calcium Gluconate Injection. Calcium ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Calcium Gluconate Injection, USP is a clear, colorless to slightly yellow solution supplied as follows: Product CodeUnit of SaleStrengthEach - PRX360019 - NDC 65219-630-19 - Unit of 25 - 1,000 ...
-
17 PATIENT COUNSELING INFORMATION
Advise the patient that the risks associated with infusion including local tissue inflammation, local necrosis and calcinosis. [see Warnings and Precautions (5.3)]. PREMIERProRx® is a ...
-
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Calcium Gluconate 10 mL
Single Dose Vial Label - NDC 65219-630-01 PRX360019 - Calcium Gluconate - Injection, USP - 1,000 mg per 10 mL - (100 mg per mL) Preservative free. 10 mL Single Dose Vial Rx ...
-
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Calcium Gluconate 10 mL
Single Dose Vial Tray Label - NDC 65219-630-19 PRX360019 - Calcium Gluconate - Injection, USP - 1,000 mg per 10 mL - (100 mg per mL) Preservative free. 25 x 10 mL - Single Dose Vials ...
-
INGREDIENTS AND APPEARANCEProduct Information