Label: RITALIN LA- methylphenidate hydrochloride capsule, extended release
- NDC Code(s): 66758-260-01, 66758-261-01, 66758-262-01, 66758-263-01
- Packager: Sandoz Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CII
- Marketing Status: New Drug Application
Drug Label Information
Updated December 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use RITALIN LA safely and effectively. See full prescribing information for RITALIN LA. RITALIN LA® (methylphenidate hydrochloride ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: ABUSE, MISUSE, AND ADDICTION
Ritalin LA has a high potential for abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Misuse and abuse of CNS stimulants, including Ritalin LA, can result in overdose and death [see Overdosage (10)], and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
Before prescribing Ritalin LA, assess each patient’s risk for abuse, misuse, and addiction. Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug. Throughout Ritalin LA treatment, reassess each patient’s risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction [see Warnings and Precautions (5.1), Drug Abuse and Dependence (9.2)].
Close -
1 INDICATIONS AND USAGERitalin LA® is indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD), in pediatric patients 6 to 12 years of age [see Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION2.1 Pretreatment Screening - Prior to treating patients with Ritalin LA, assess: • for the presence of cardiac disease (i.e., perform a careful history, family history of sudden death or ...
-
3 DOSAGE FORMS AND STRENGTHS• 10 mg extended-release capsules white/light brown, (imprinted "SDZ R10") • 20 mg extended-release capsules white, (imprinted "SDZ R20") • 30 mg extended-release capsules yellow, (imprinted "SDZ ...
-
4 CONTRAINDICATIONS• Hypersensitivity to methylphenidate or other components of Ritalin LA. Hypersensitivity reactions, such as angioedema and anaphylactic reactions, have been reported in patients treated with ...
-
5 WARNINGS AND PRECAUTIONS5.1 Abuse, Misuse, and Addiction - Ritalin LA has a high potential for abuse and misuse. The use of Ritalin LA exposes individuals to the risks of abuse and misuse, which can lead to the ...
-
6 ADVERSE REACTIONSThe following are discussed in more detail in other sections of the labeling: • Abuse, Misuse, and Addiction [see Boxed Warning, Warnings and Precautions (5.1), Drug Abuse and Dependence (9.2 ...
-
7 DRUG INTERACTIONS7.1 Clinically Important Drug Interactions with Ritalin LA - Table 3 presents clinically important drug interactions with Ritalin LA. Table 3: Clinically Important Drug Interactions with ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ADHD medications, including Ritalin LA during ...
-
9 DRUG ABUSE AND DEPENDENCE9.1 Controlled Substance - Ritalin LA contains methylphenidate hydrochloride, a Schedule II controlled substance. 9.2 Abuse - Ritalin LA has a high potential for abuse and misuse which ...
-
10 OVERDOSAGEClinical Effects of Overdose - Overdose of CNS stimulants is characterized by the following sympathomimetic effects: • Cardiovascular effects including tachyarrhythmias, and hypertension or ...
-
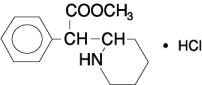
11 DESCRIPTIONRitalin LA contains methylphenidate hydrochloride, a CNS stimulant. Ritalin LA extended-release capsules is an extended-release formulation of methylphenidate for oral administration with a ...
-
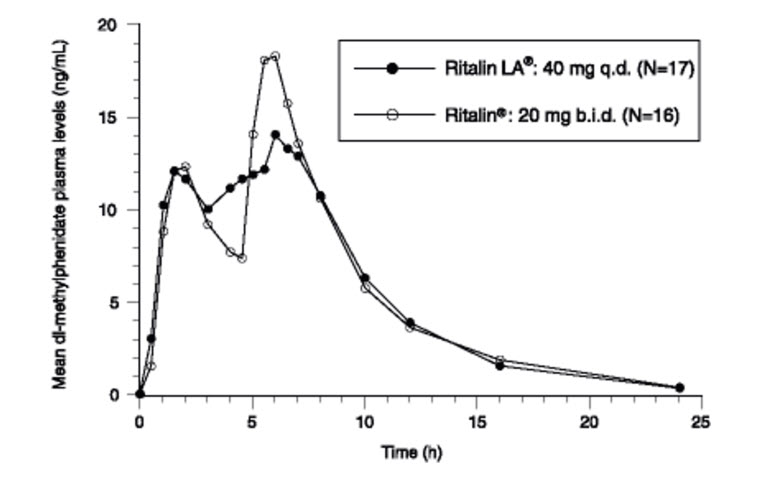
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Methylphenidate hydrochloride is a CNS stimulant. The mode of therapeutic action in ADHD is not known. 12.2 Pharmacodynamics - Methylphenidate is a racemic ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility - Carcinogenesis - In a lifetime carcinogenicity study carried out in B6C3F1 mice, methylphenidate caused an increase in ...
-
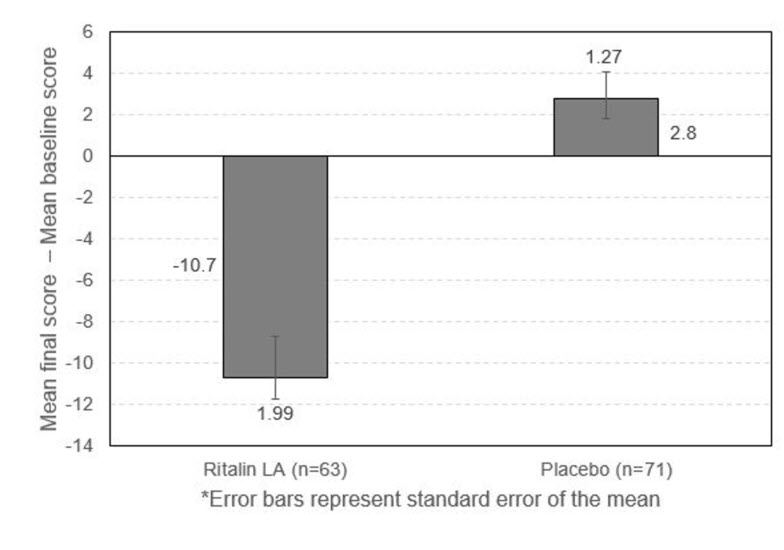
14 CLINICAL STUDIES14.1 Children and Adolescents - Ritalin LA was evaluated in a randomized, double-blind, placebo-controlled, parallel group clinical study in which 134 children, ages 6 to 12 years, with ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING• 10 mg extended-release capsules (NDC 66758-260-01) white/light brown, (imprinted “SDZ R10”) supplied in bottles of 100 - • 20 mg extended-release capsules (NDC 66758-261-01) white, (imprinted ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Abuse, Misuse, and Addiction - Educate patients and their families about the risks of abuse, misuse, and addiction ...
-
MEDICATION GUIDEMEDICATION GUIDE - RITALIN LA® (rit-ah-lin LA) (methylphenidate hydrochloride) extended-release capsules for oral use, CII - What is the most important information I should know about ...
-
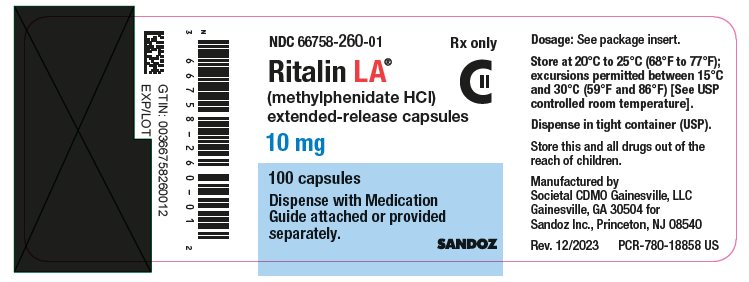
PRINCIPAL DISPLAY PANELNDC 66758-260-01 Rx only - Ritalin LA® (methylphenidate HCl) extended-release capsules - 10 mg - 100 capsules - Dispense with Medication - Guide attached or provided - separately. SANDOZ
-
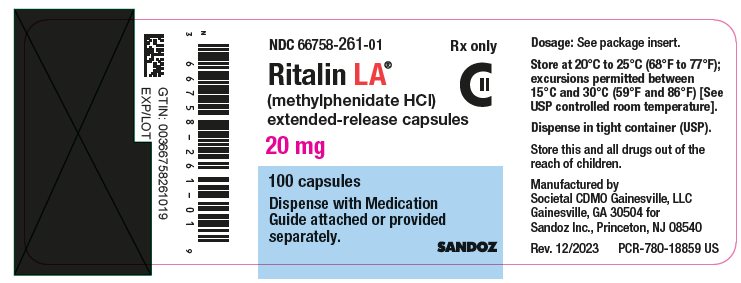
PRINCIPAL DISPLAY PANELNDC 66758-261-01 Rx only - Ritalin LA® (methylphenidate HCl) extended-release capsules - 20 mg - 100 capsules - Dispense with Medication - Guide attached or provided - separately. SANDOZ
-
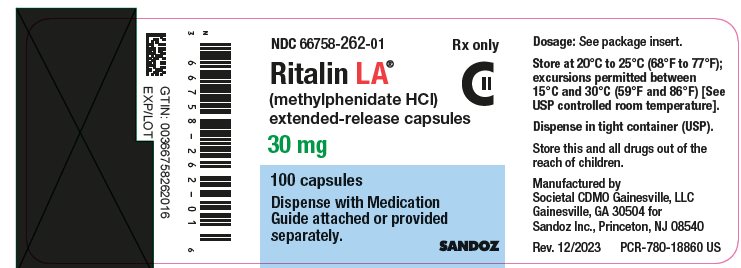
PRINCIPAL DISPLAY PANELNDC 66758-262-01 Rx only - Ritalin LA® (methylphenidate HCl) extended-release capsules - 30 mg - 100 capsules - Dispense with Medication - Guide attached or provided - separately. SANDOZ
-
PRINCIPAL DISPLAY PANELNDC 66758-263-01 Rx only - Ritalin LA® (methylphenidate HCl) extended-release capsules - 40 mg - 100 capsules - Dispense with Medication - Guide attached or provided - separately. SANDOZ
-
INGREDIENTS AND APPEARANCEProduct Information