Label: HEPLISAV-B (hepatitis b vaccine- recombinant adjuvanted injection, solution
- NDC Code(s): 83703-048-01, 83703-048-05
- Packager: Bamboo US BidCo LLC
- Category: VACCINE LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated June 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
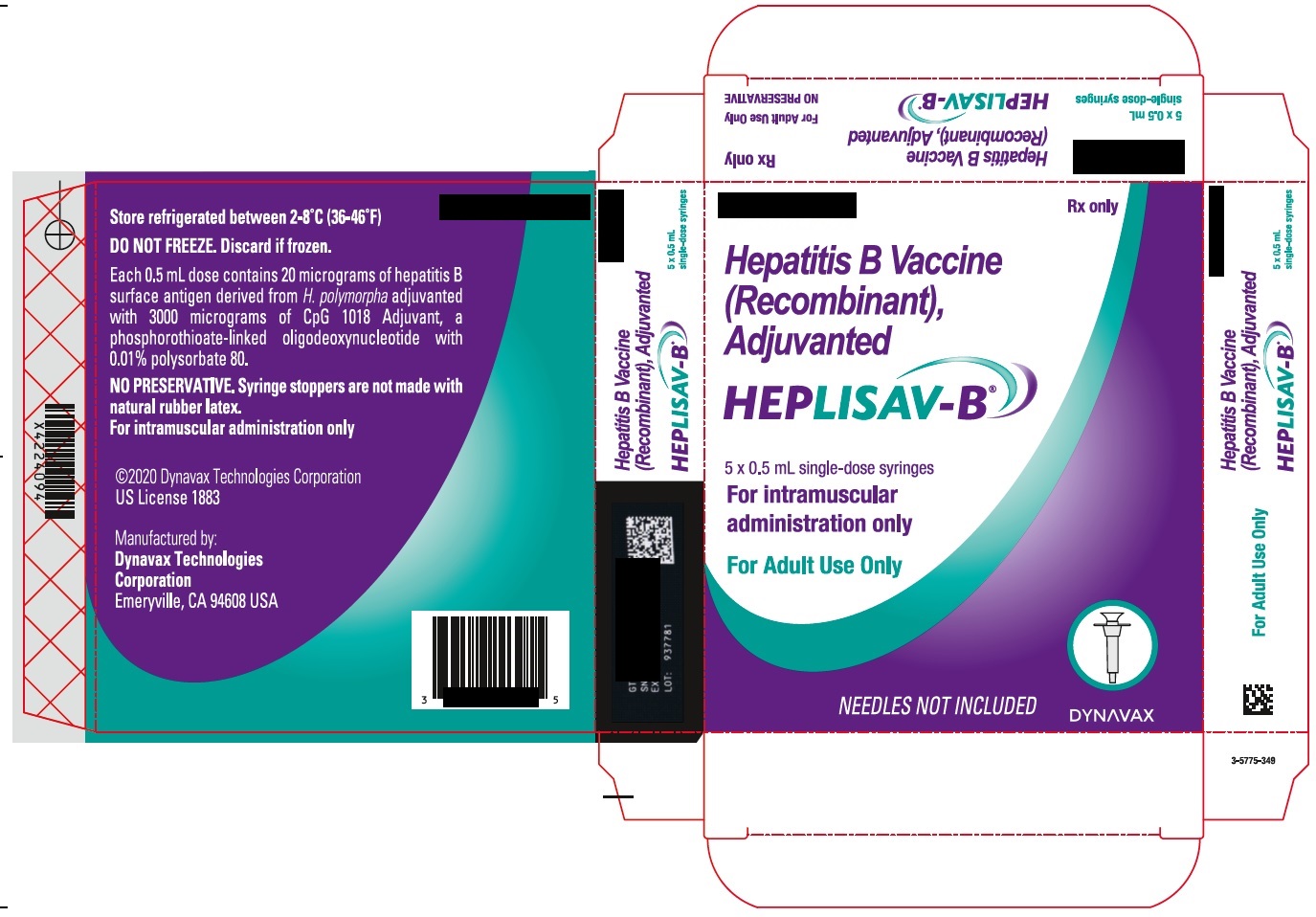
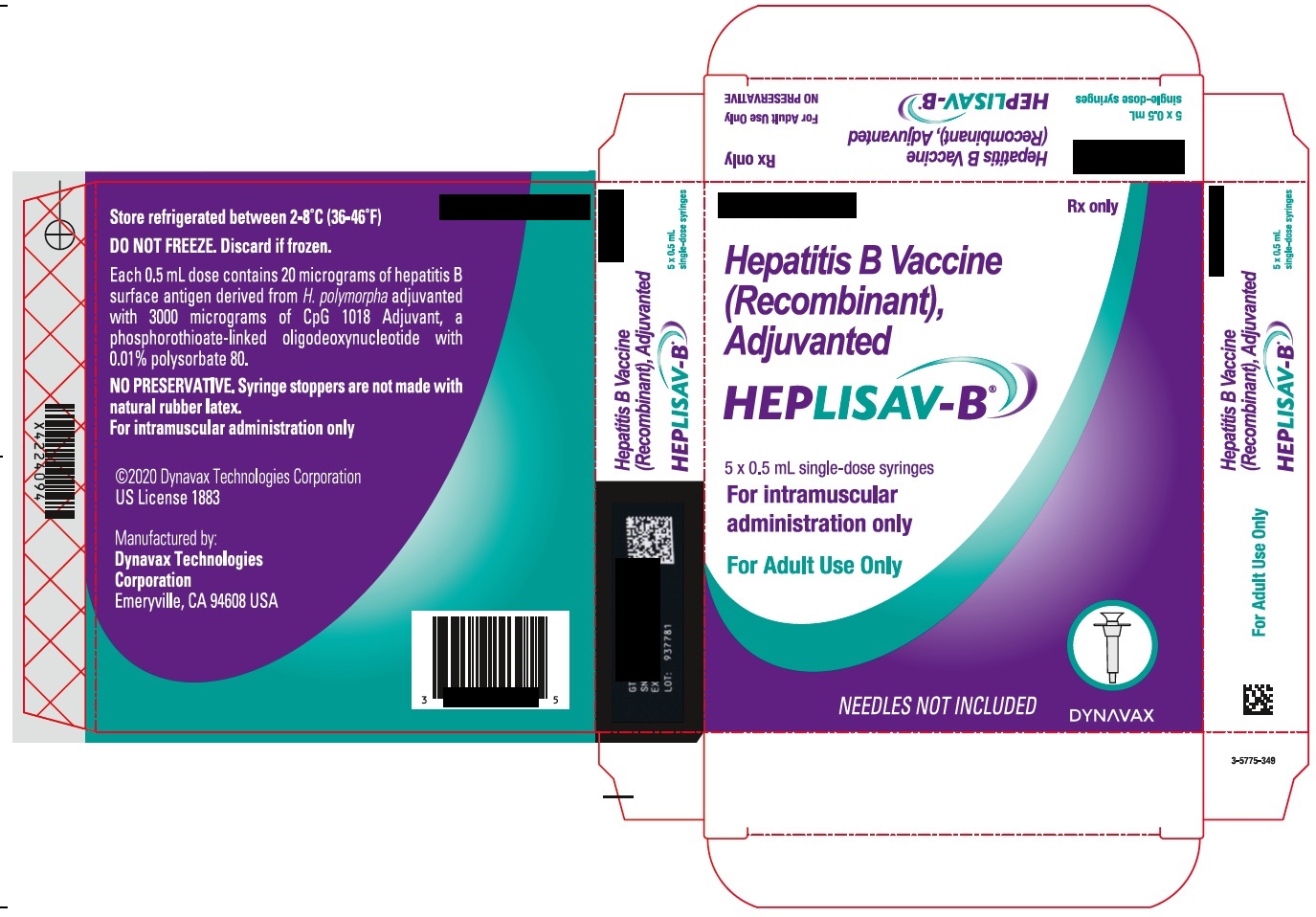
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HEPLISAV-B
hepatitis b vaccine (recombinant) adjuvanted injection, solutionProduct Information Product Type VACCINE Item Code (Source) NDC:83703-048 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HEPATITIS B VIRUS SUBTYPE ADW HBSAG SURFACE PROTEIN ANTIGEN (UNII: XL4HLC6JH6) (HEPATITIS B VIRUS SUBTYPE ADW HBSAG SURFACE PROTEIN ANTIGEN - UNII:XL4HLC6JH6) HEPATITIS B VIRUS SUBTYPE ADW HBSAG SURFACE PROTEIN ANTIGEN 20 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength 1018 ISS (UNII: 25DT549L0G) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC, DODECAHYDRATE (UNII: E1W4N241FO) SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83703-048-05 5 in 1 CARTON 1 NDC:83703-048-01 0.5 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125428 01/01/2018 Labeler - Bamboo US BidCo LLC (119087615) Registrant - Dynavax Technologies Corporation (964173801)