Label: VAQTA- hepatitis a vaccine, inactivated injection, suspension
- NDC Code(s): 83703-045-01, 83703-045-02, 83703-046-01, 83703-046-02
- Packager: Bamboo US BidCo LLC
- Category: VACCINE LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated June 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PRINCIPAL DISPLAY PANEL - 0.5 mL Vials Carton
10 Single-dose ~25U/0.5-mL Vials
(HEPATITIS A VACCINE, INACTIVATED)
VAQTA ®
PEDIATRIC/ADOLESCENT0.5 mL vial contains approximately 25U of hepatitis A virus antigen on an Amorphous aluminum
hydroxyphosphate sulfate adjuvant. Hepatitis A virus is grown in cell culture in human MRC-5
diploid fibroblasts.Rx only

-
PRINCIPAL DISPLAY PANEL - 0.5 mL Syringes Carton
10 Single-Dose 0.5-mL Syringes[HEPATITIS A VACCINE,
INACTIVATED]
VAQTA ®PEDIATRIC/ADOLESCENT
FORMULATIONThis package contains 10 single-dose syringes.
Each 0.5-mL syringe contains approximately 25U of hepatitis A virus antigen
on an amorphous aluminum hydroxyphosphate sulfate adjuvant.Hepatitis A virus is grown in cell culture in human MRC-5 diploid fibroblasts.
Rx only

-
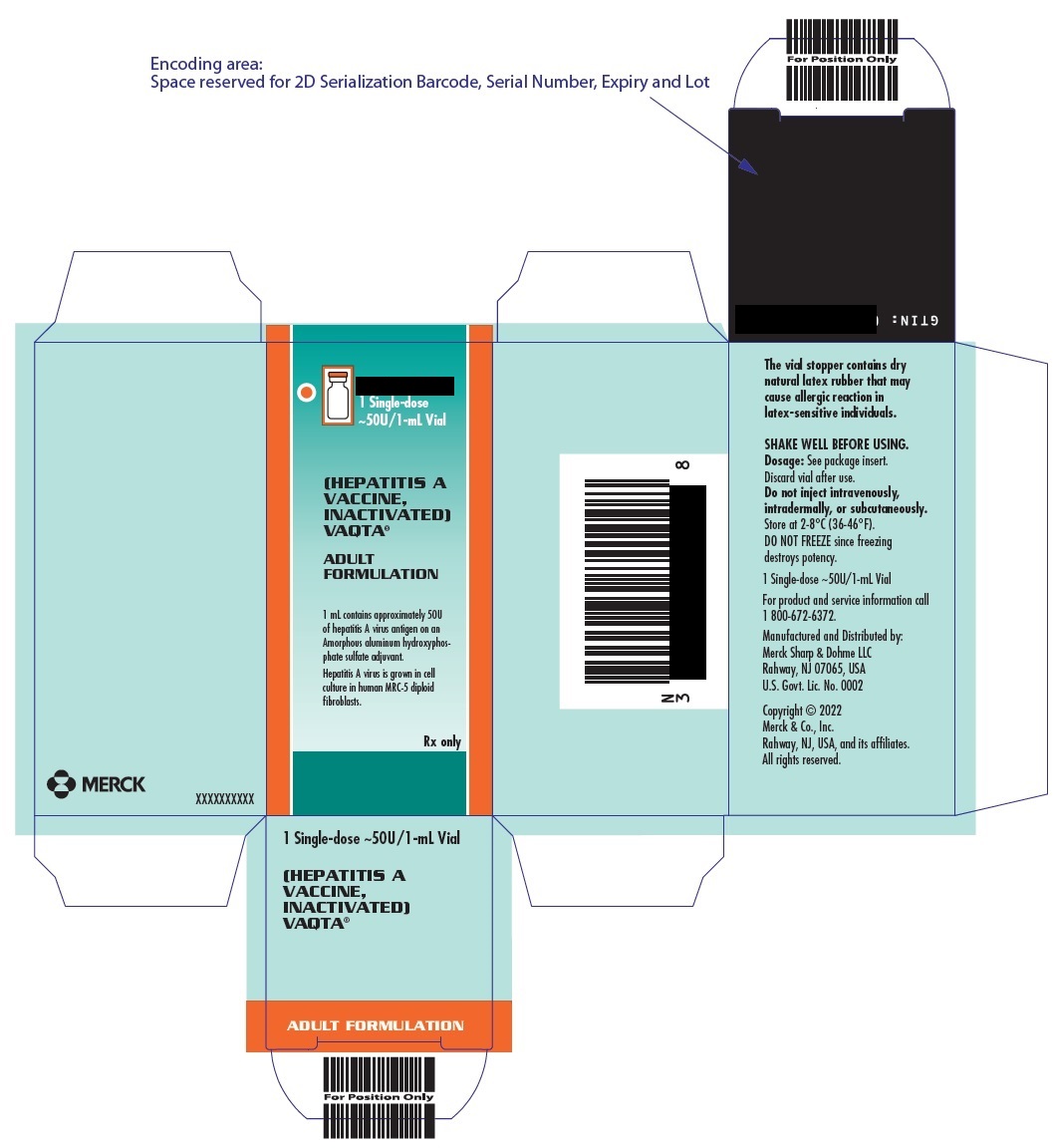
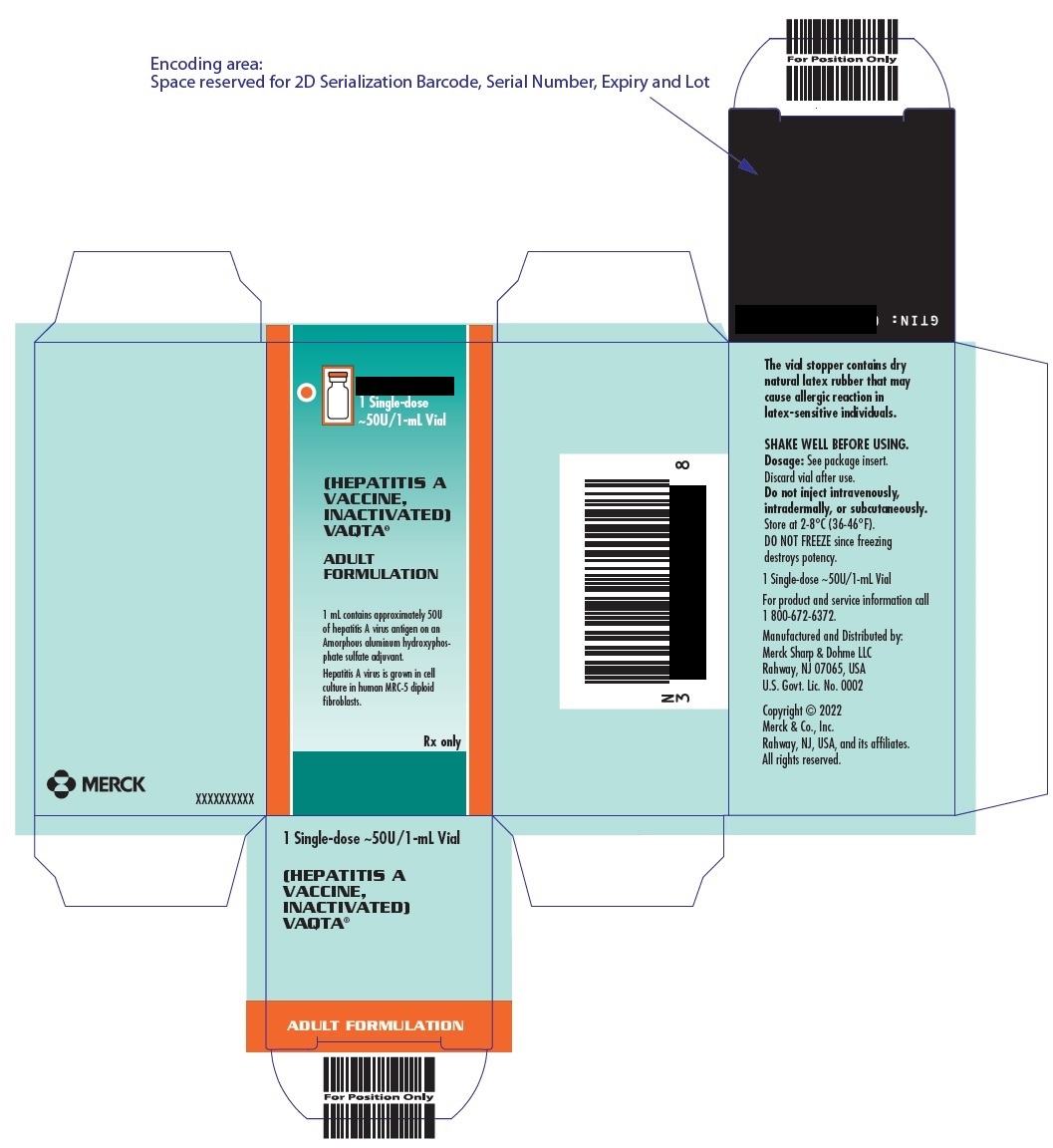
PRINCIPAL DISPLAY PANEL - 1 mL Vials Carton
10 Single-dose
~50U/1-mL Vials(HEPATITIS A VACCINE, INACTIVATED)
VAQTA ®
ADULT FORMULATION1 mL vial contains approximately 50U of hepatitis A virus antigen on an Amorphous aluminum
hydroxyphosphate sulfate adjuvant. Hepatitis A virus is grown in cell culture in human MRC-5
diploid fibroblasts.Rx only

-
PRINCIPAL DISPLAY PANEL - 1 mL Syringes Carton
10 Single-Dose 1-mL Syringes
[HEPATITIS A VACCINE,
INACTIVATED]
VAQTA ®ADULT FORMULATION
This package contains 10 single-dose syringes.
Each 1-mL syringe contains approximately 50U of hepatitis A virus antigen
on an amorphous aluminum hydroxyphosphate sulfate adjuvant.Hepatitis A virus is grown in cell culture in human MRC-5 diploid fibroblasts.
Rx only

- PRINCIPAL DISPLAY PANEL - 1 mL Vial Carton
-
INGREDIENTS AND APPEARANCE
VAQTA
hepatitis a vaccine, inactivated injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:83703-045 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HEPATITIS A VIRUS STRAIN CR 326F ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: Q04Q922K9Q) (HEPATITIS A VIRUS STRAIN CR 326F ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:Q04Q922K9Q) HEPATITIS A VIRUS STRAIN CR 326F ANTIGEN (FORMALDEHYDE INACTIVATED) 25 [iU] in 0.5 mL Inactive Ingredients Ingredient Name Strength SODIUM BORATE (UNII: 91MBZ8H3QO) 35 ug in 0.5 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Product Characteristics Color white (slightly opaque, white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83703-045-02 10 in 1 CARTON 1 NDC:83703-045-01 0.5 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103606 03/29/1996 VAQTA
hepatitis a vaccine, inactivated injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:83703-046 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HEPATITIS A VIRUS STRAIN CR 326F ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: Q04Q922K9Q) (HEPATITIS A VIRUS STRAIN CR 326F ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:Q04Q922K9Q) HEPATITIS A VIRUS STRAIN CR 326F ANTIGEN (FORMALDEHYDE INACTIVATED) 50 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM BORATE (UNII: 91MBZ8H3QO) 70 ug in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Product Characteristics Color white (slightly opaque, white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83703-046-02 10 in 1 CARTON 1 NDC:83703-046-01 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103606 03/29/1996 Labeler - Bamboo US BidCo LLC (119087615)