Label: GARDASIL 9- human papillomavirus 9-valent vaccine, recombinant injection, suspension
- NDC Code(s): 83703-047-01, 83703-047-02
- Packager: Bamboo US BidCo LLC
- Category: VACCINE LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated June 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
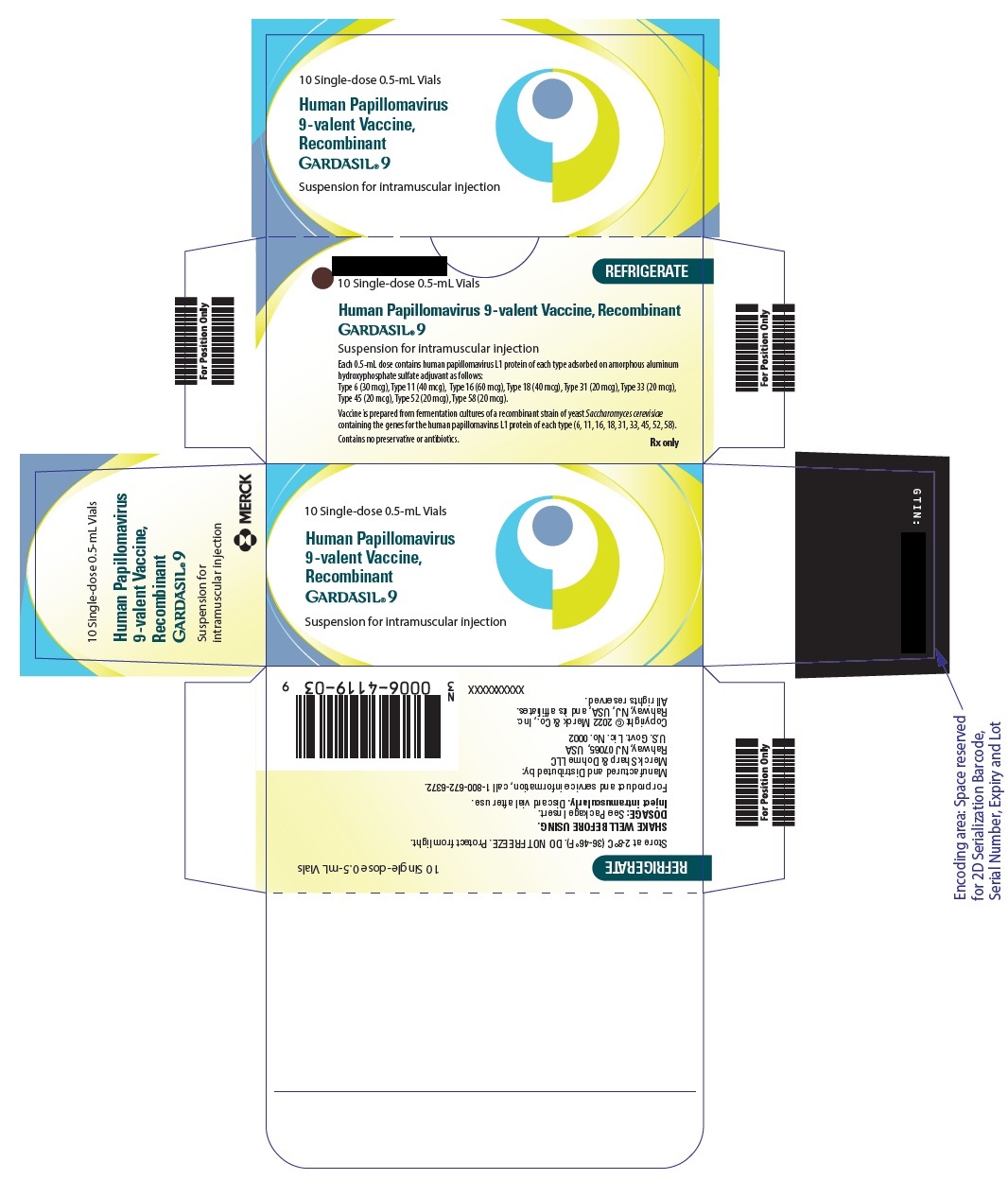
PRINCIPAL DISPLAY PANEL - 0.5 mL Vial Carton
10 Single-dose 0.5-mL Vials
REFRIGERATE
Human Papillomavirus 9-valent Vaccine, Recombinant
GARDASIL ®9
Suspension for intramuscular injection
Each 0.5-mL dose contains human papillomavirus L1 protein of each type adsorbed on amorphous aluminum
hydroxyphosphate sulfate adjuvant as follows:
Type 6 (30 mcg), Type 11 (40 mcg), Type 16 (60 mcg), Type 18 (40 mcg), Type 31 (20 mcg), Type 33 (20 mcg),
Type 45 (20 mcg), Type 52 (20 mcg), Type 58 (20 mcg).Vaccine is prepared from fermentation cultures of a recombinant strain of yeast Saccharomyces cerevisiae
containing the genes for the human papillomavirus L1 protein of each type (6, 11, 16, 18, 31, 33, 45, 52, 58).Contains no preservative or antibiotics.
Rx only
-
PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe Carton
10 Single-dose 0.5-mL Syringes
REFRIGERATE
Human Papillomavirus
9-valent Vaccine, RecombinantGARDASIL ®9
Suspension for intramuscular injection
Each 0.5-mL dose contains human papillomavirus L1 protein of each type
adsorbed on amorphous aluminum hydroxyphosphate sulfate adjuvant
as follows:
Type 6 (30 mcg), Type 11 (40 mcg), Type 16 (60 mcg), Type 18 (40 mcg),
Type 31 (20 mcg), Type 33 (20 mcg), Type 45 (20 mcg), Type 52 (20 mcg),
Type 58 (20 mcg).Vaccine is prepared from fermentation cultures of a recombinant strain of yeast
Saccharomyces cerevisiaecontaining the genes for the human papillomavirus
L1 protein of each type (6, 11, 16, 18, 31, 33, 45, 52, 58).Contains no preservative or antibiotics.
Rx only

-
INGREDIENTS AND APPEARANCE
GARDASIL 9
human papillomavirus 9-valent vaccine, recombinant injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:83703-047 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN PAPILLOMAVIRUS TYPE 6 L1 CAPSID PROTEIN ANTIGEN (UNII: 61746O90DY) (HUMAN PAPILLOMAVIRUS TYPE 6 L1 CAPSID PROTEIN ANTIGEN - UNII:61746O90DY) HUMAN PAPILLOMAVIRUS TYPE 6 L1 CAPSID PROTEIN ANTIGEN 30 ug in 0.5 mL HUMAN PAPILLOMAVIRUS TYPE 11 L1 CAPSID PROTEIN ANTIGEN (UNII: Z845VHQ61P) (HUMAN PAPILLOMAVIRUS TYPE 11 L1 CAPSID PROTEIN ANTIGEN - UNII:Z845VHQ61P) HUMAN PAPILLOMAVIRUS TYPE 11 L1 CAPSID PROTEIN ANTIGEN 40 ug in 0.5 mL HUMAN PAPILLOMAVIRUS TYPE 16 L1 CAPSID PROTEIN ANTIGEN (UNII: 6LTE2DNX63) (HUMAN PAPILLOMAVIRUS TYPE 16 L1 CAPSID PROTEIN ANTIGEN - UNII:6LTE2DNX63) HUMAN PAPILLOMAVIRUS TYPE 16 L1 CAPSID PROTEIN ANTIGEN 60 ug in 0.5 mL HUMAN PAPILLOMAVIRUS TYPE 18 L1 CAPSID PROTEIN ANTIGEN (UNII: J2D279PEM5) (HUMAN PAPILLOMAVIRUS TYPE 18 L1 CAPSID PROTEIN ANTIGEN - UNII:J2D279PEM5) HUMAN PAPILLOMAVIRUS TYPE 18 L1 CAPSID PROTEIN ANTIGEN 40 ug in 0.5 mL HUMAN PAPILLOMAVIRUS TYPE 31 L1 CAPSID PROTEIN ANTIGEN (UNII: 53JIL371NS) (HUMAN PAPILLOMAVIRUS TYPE 31 L1 CAPSID PROTEIN ANTIGEN - UNII:53JIL371NS) HUMAN PAPILLOMAVIRUS TYPE 31 L1 CAPSID PROTEIN ANTIGEN 20 ug in 0.5 mL HUMAN PAPILLOMAVIRUS TYPE 33 L1 CAPSID PROTEIN ANTIGEN (UNII: 759RAC446C) (HUMAN PAPILLOMAVIRUS TYPE 33 L1 CAPSID PROTEIN ANTIGEN - UNII:759RAC446C) HUMAN PAPILLOMAVIRUS TYPE 33 L1 CAPSID PROTEIN ANTIGEN 20 ug in 0.5 mL HUMAN PAPILLOMAVIRUS TYPE 45 L1 CAPSID PROTEIN ANTIGEN (UNII: 68S8VCN34F) (HUMAN PAPILLOMAVIRUS TYPE 45 L1 CAPSID PROTEIN ANTIGEN - UNII:68S8VCN34F) HUMAN PAPILLOMAVIRUS TYPE 45 L1 CAPSID PROTEIN ANTIGEN 20 ug in 0.5 mL HUMAN PAPILLOMAVIRUS TYPE 52 L1 CAPSID PROTEIN ANTIGEN (UNII: 55644W68FD) (HUMAN PAPILLOMAVIRUS TYPE 52 L1 CAPSID PROTEIN ANTIGEN - UNII:55644W68FD) HUMAN PAPILLOMAVIRUS TYPE 52 L1 CAPSID PROTEIN ANTIGEN 20 ug in 0.5 mL HUMAN PAPILLOMAVIRUS TYPE 58 L1 CAPSID PROTEIN ANTIGEN (UNII: 94Y15HP7LF) (HUMAN PAPILLOMAVIRUS TYPE 58 L1 CAPSID PROTEIN ANTIGEN - UNII:94Y15HP7LF) HUMAN PAPILLOMAVIRUS TYPE 58 L1 CAPSID PROTEIN ANTIGEN 20 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 9.56 mg in 0.5 mL HISTIDINE (UNII: 4QD397987E) 0.78 mg in 0.5 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 50 ug in 0.5 mL SODIUM BORATE (UNII: 91MBZ8H3QO) 35 ug in 0.5 mL WATER (UNII: 059QF0KO0R) Product Characteristics Color white (white, cloudy) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83703-047-02 10 in 1 CARTON 1 NDC:83703-047-01 0.5 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125508 12/10/2014 Labeler - Bamboo US BidCo LLC (119087615)