Label: HYQVIA (immune globulin infusion 10%- human with recombinant human hyaluronidase kit
-
NDC Code(s):
0944-2715-25,
0944-2716-05,
0944-2717-10,
0944-2718-20, view more0944-2719-30, 0944-2720-03, 0944-2721-03, 0944-2722-03, 0944-2723-03, 0944-2724-03, 83703-038-02, 83703-039-02, 83703-040-02, 83703-041-02, 83703-042-02
- Packager: Bamboo US BidCo LLC
- Category: PLASMA DERIVATIVE
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL - 2.5 g/25 mL Vial Label
- PRINCIPAL DISPLAY PANEL - 1.25 mL Vial Label
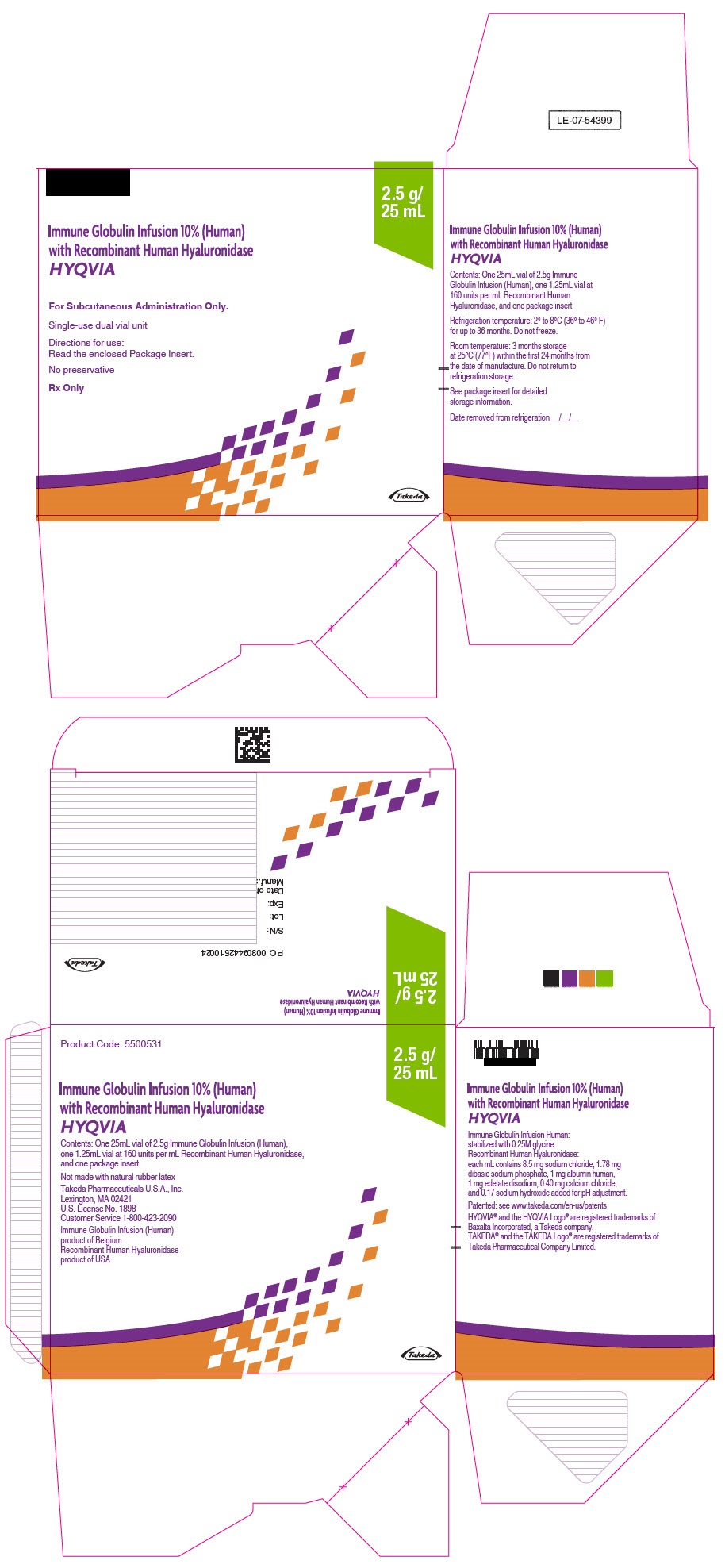
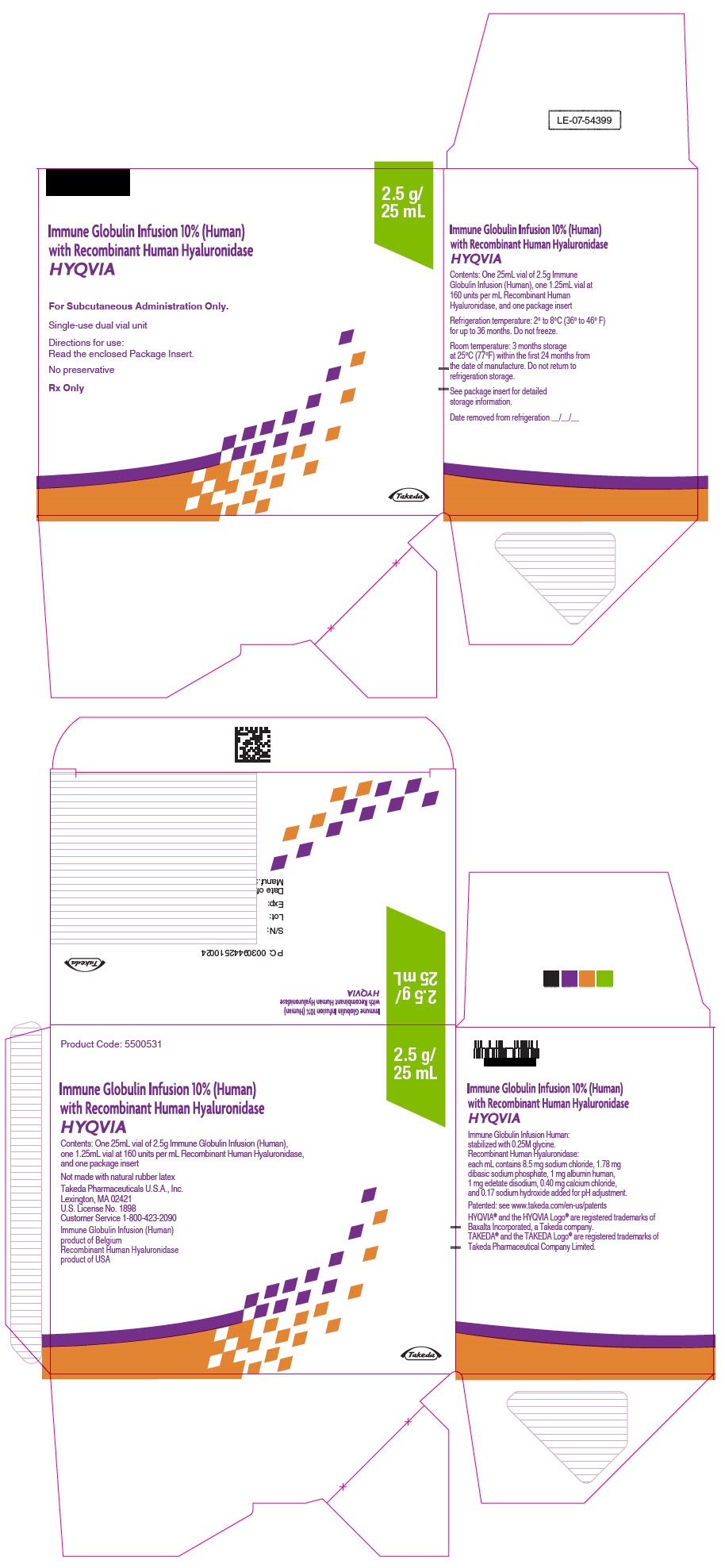
- PRINCIPAL DISPLAY PANEL - 2.5 g/25 mL Kit Carton
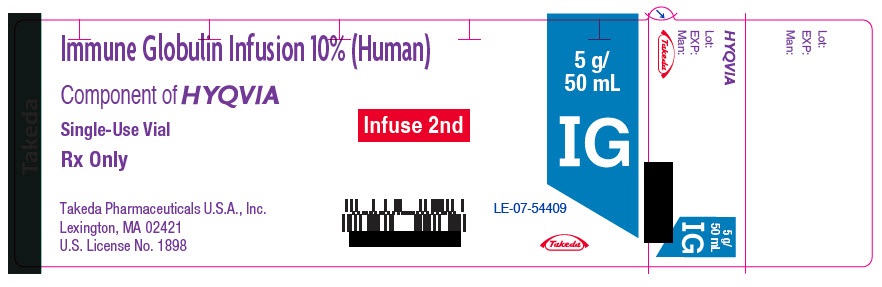
- PRINCIPAL DISPLAY PANEL - 5 g/50 mL Vial Label
- PRINCIPAL DISPLAY PANEL - 2.5 mL Vial Label
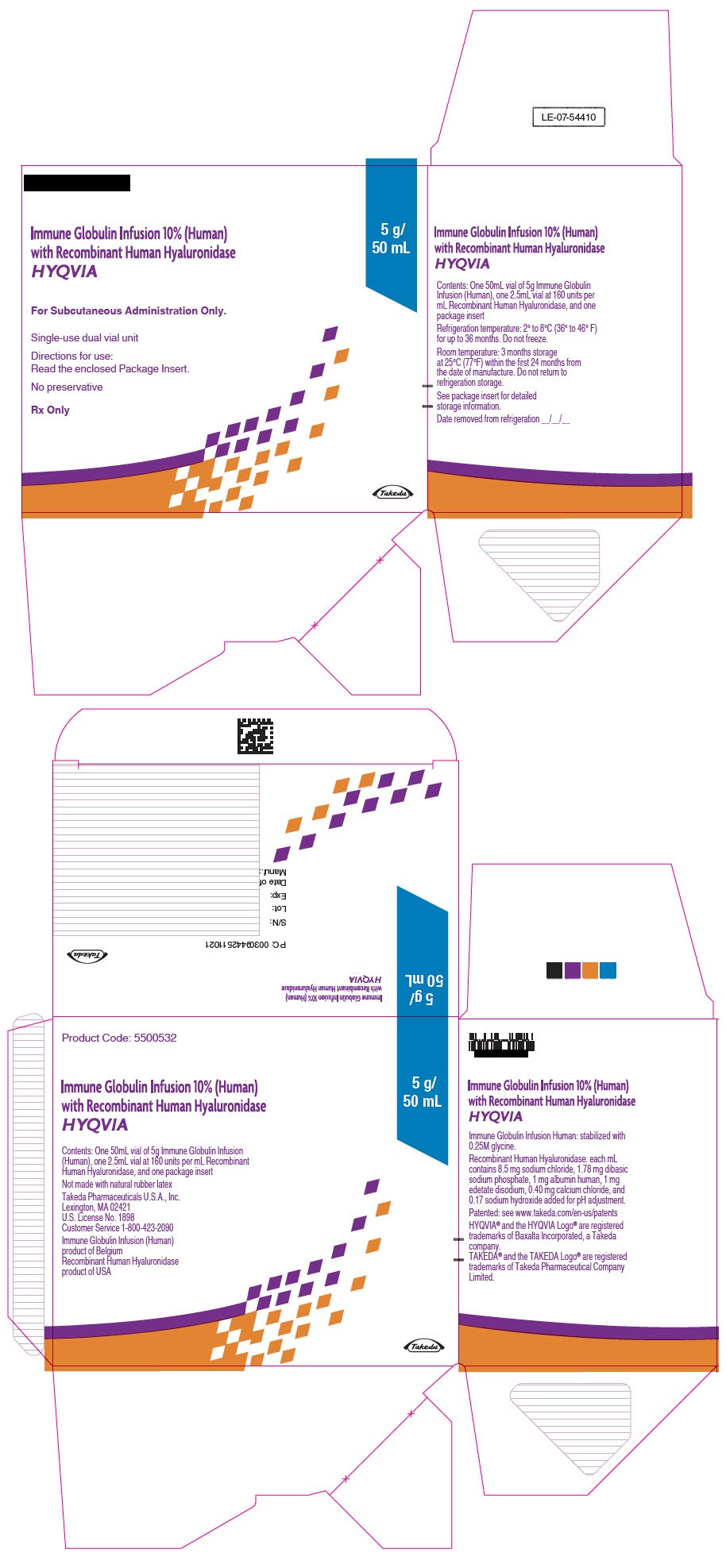
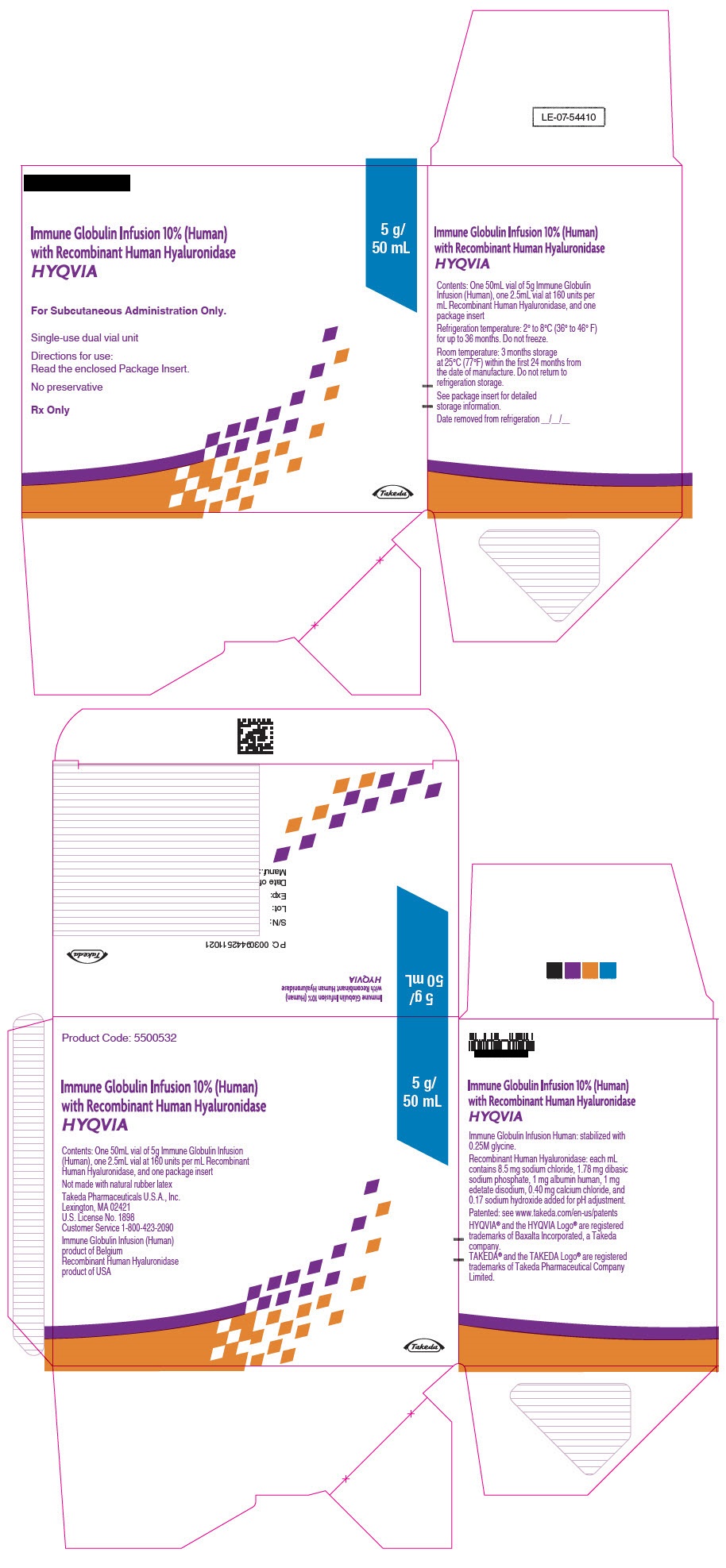
- PRINCIPAL DISPLAY PANEL - 5 g/50 mL Kit Carton
- PRINCIPAL DISPLAY PANEL - 10 g/100 mL Vial Label
- PRINCIPAL DISPLAY PANEL - 5 mL Vial Label
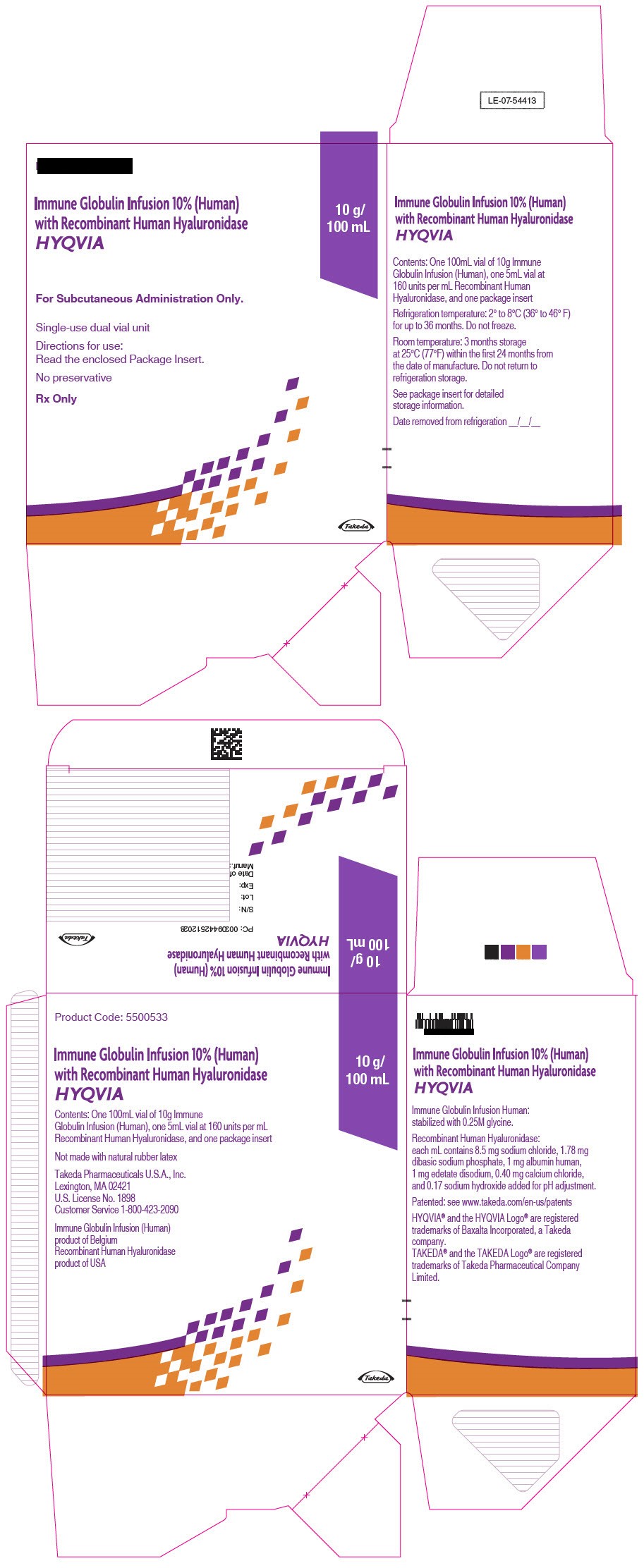
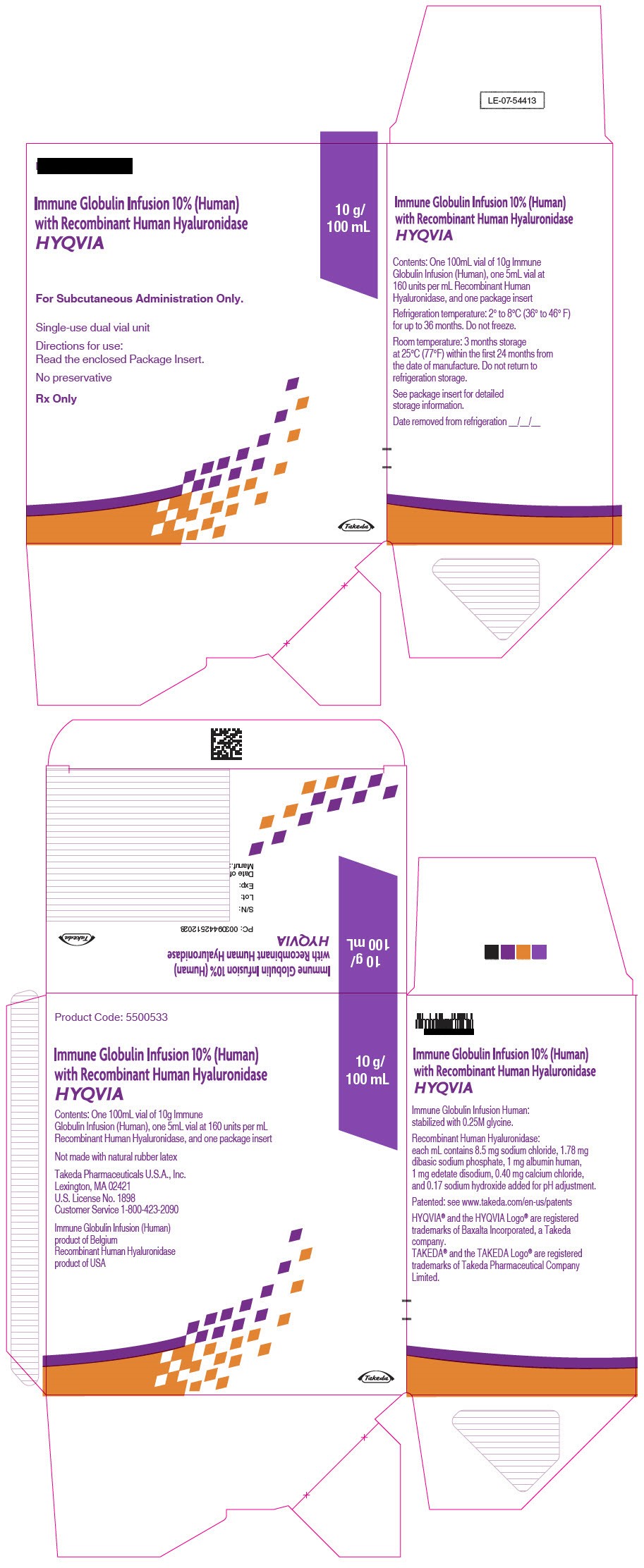
- PRINCIPAL DISPLAY PANEL - 10 g/100 mL Kit Carton
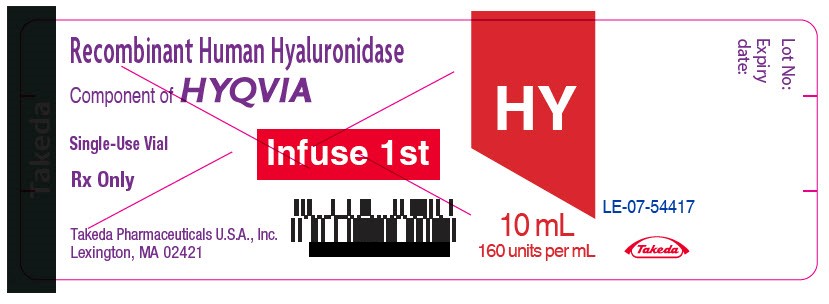
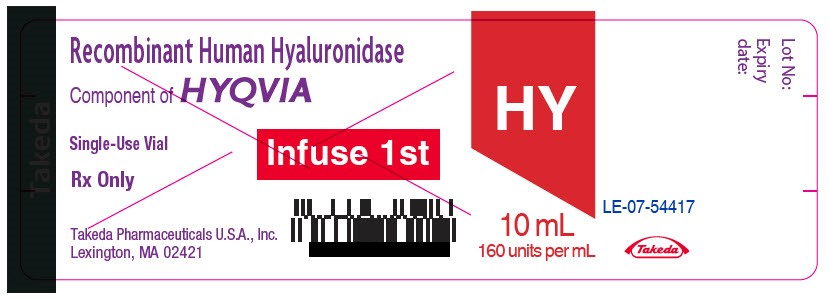
- PRINCIPAL DISPLAY PANEL - 10 mL Vial Label
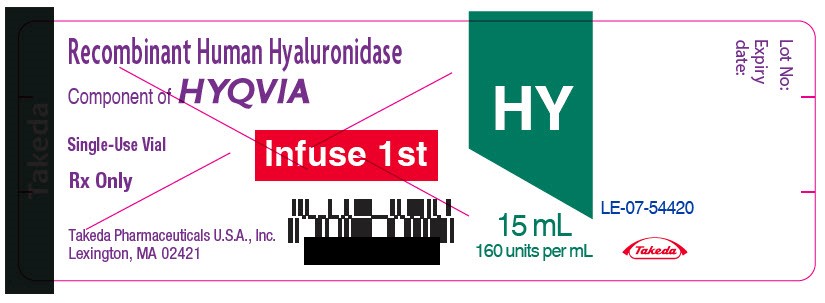
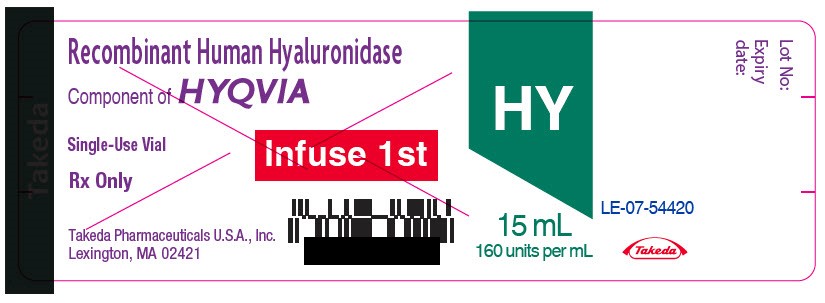
- PRINCIPAL DISPLAY PANEL - 15 mL Vial Label
-
INGREDIENTS AND APPEARANCE
HYQVIA
immune globulin infusion 10% (human) with recombinant human hyaluronidase kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:83703-039 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83703-039-02 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 50 mL Part 2 1 VIAL, GLASS 2.5 mL Part 1 of 2 IMMUNE GLOBULIN INFUSION (HUMAN), 10%
human immuneglobulin g solutionProduct Information Item Code (Source) NDC:0944-2716 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN IMMUNOGLOBULIN G (UNII: 66Y330CJHS) (HUMAN IMMUNOGLOBULIN G - UNII:66Y330CJHS) HUMAN IMMUNOGLOBULIN G 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCINE (UNII: TE7660XO1C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-2716-05 50 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 06/04/2012 Part 2 of 2 RECOMBINANT HUMAN HYALURONIDASE
hyaluronidase (human recombinant) solutionProduct Information Item Code (Source) NDC:0944-2721 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYALURONIDASE (HUMAN RECOMBINANT) (UNII: 743QUY4VD8) (HYALURONIDASE (HUMAN RECOMBINANT) - UNII:743QUY4VD8) HYALURONIDASE (HUMAN RECOMBINANT) 160 [USP'U] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE (UNII: SE337SVY37) ALBUMIN HUMAN (UNII: ZIF514RVZR) EDETATE DISODIUM (UNII: 7FLD91C86K) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-2721-03 2.5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 09/12/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 09/12/2014 HYQVIA
immune globulin infusion 10% (human) with recombinant human hyaluronidase kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:83703-040 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83703-040-02 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 100 mL Part 2 1 VIAL, GLASS 5 mL Part 1 of 2 IMMUNE GLOBULIN INFUSION (HUMAN), 10%
human immuneglobulin g solutionProduct Information Item Code (Source) NDC:0944-2717 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN IMMUNOGLOBULIN G (UNII: 66Y330CJHS) (HUMAN IMMUNOGLOBULIN G - UNII:66Y330CJHS) HUMAN IMMUNOGLOBULIN G 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCINE (UNII: TE7660XO1C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-2717-10 100 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 06/04/2012 Part 2 of 2 RECOMBINANT HUMAN HYALURONIDASE
hyaluronidase (human recombinant) solutionProduct Information Item Code (Source) NDC:0944-2722 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYALURONIDASE (HUMAN RECOMBINANT) (UNII: 743QUY4VD8) (HYALURONIDASE (HUMAN RECOMBINANT) - UNII:743QUY4VD8) HYALURONIDASE (HUMAN RECOMBINANT) 160 [USP'U] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE (UNII: SE337SVY37) ALBUMIN HUMAN (UNII: ZIF514RVZR) EDETATE DISODIUM (UNII: 7FLD91C86K) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-2722-03 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 09/12/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 09/12/2014 HYQVIA
immune globulin infusion 10% (human) with recombinant human hyaluronidase kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:83703-041 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83703-041-02 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 200 mL Part 2 1 VIAL, GLASS 10 mL Part 1 of 2 IMMUNE GLOBULIN INFUSION (HUMAN), 10%
human immuneglobulin g solutionProduct Information Item Code (Source) NDC:0944-2718 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN IMMUNOGLOBULIN G (UNII: 66Y330CJHS) (HUMAN IMMUNOGLOBULIN G - UNII:66Y330CJHS) HUMAN IMMUNOGLOBULIN G 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCINE (UNII: TE7660XO1C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-2718-20 200 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 06/04/2012 Part 2 of 2 RECOMBINANT HUMAN HYALURONIDASE
hyaluronidase (human recombinant) solutionProduct Information Item Code (Source) NDC:0944-2723 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYALURONIDASE (HUMAN RECOMBINANT) (UNII: 743QUY4VD8) (HYALURONIDASE (HUMAN RECOMBINANT) - UNII:743QUY4VD8) HYALURONIDASE (HUMAN RECOMBINANT) 160 [USP'U] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE (UNII: SE337SVY37) ALBUMIN HUMAN (UNII: ZIF514RVZR) EDETATE DISODIUM (UNII: 7FLD91C86K) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-2723-03 10 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 09/12/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 09/12/2014 HYQVIA
immune globulin infusion 10% (human) with recombinant human hyaluronidase kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:83703-038 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83703-038-02 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 25 mL Part 2 1 VIAL, GLASS 1.25 mL Part 1 of 2 IMMUNE GLOBULIN INFUSION (HUMAN), 10%
human immuneglobulin g solutionProduct Information Item Code (Source) NDC:0944-2715 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN IMMUNOGLOBULIN G (UNII: 66Y330CJHS) (HUMAN IMMUNOGLOBULIN G - UNII:66Y330CJHS) HUMAN IMMUNOGLOBULIN G 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCINE (UNII: TE7660XO1C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-2715-25 25 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 09/12/2014 Part 2 of 2 RECOMBINANT HUMAN HYALURONIDASE
hyaluronidase (human recombinant) solutionProduct Information Item Code (Source) NDC:0944-2720 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYALURONIDASE (HUMAN RECOMBINANT) (UNII: 743QUY4VD8) (HYALURONIDASE (HUMAN RECOMBINANT) - UNII:743QUY4VD8) HYALURONIDASE (HUMAN RECOMBINANT) 160 [USP'U] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE (UNII: SE337SVY37) ALBUMIN HUMAN (UNII: ZIF514RVZR) EDETATE DISODIUM (UNII: 7FLD91C86K) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-2720-03 1.25 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 09/12/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 09/12/2014 12/17/2024 HYQVIA
immune globulin infusion 10% (human) with recombinant human hyaluronidase kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:83703-042 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83703-042-02 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 300 mL Part 2 1 VIAL, GLASS 15 mL Part 1 of 2 IMMUNE GLOBULIN INFUSION (HUMAN), 10%
human immuneglobulin g solutionProduct Information Item Code (Source) NDC:0944-2719 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN IMMUNOGLOBULIN G (UNII: 66Y330CJHS) (HUMAN IMMUNOGLOBULIN G - UNII:66Y330CJHS) HUMAN IMMUNOGLOBULIN G 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCINE (UNII: TE7660XO1C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-2719-30 300 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 06/04/2012 Part 2 of 2 RECOMBINANT HUMAN HYALURONIDASE
hyaluronidase (human recombinant) solutionProduct Information Item Code (Source) NDC:0944-2724 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYALURONIDASE (HUMAN RECOMBINANT) (UNII: 743QUY4VD8) (HYALURONIDASE (HUMAN RECOMBINANT) - UNII:743QUY4VD8) HYALURONIDASE (HUMAN RECOMBINANT) 160 [USP'U] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE (UNII: SE337SVY37) ALBUMIN HUMAN (UNII: ZIF514RVZR) EDETATE DISODIUM (UNII: 7FLD91C86K) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-2724-03 15 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 09/12/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125402 09/12/2014 Labeler - Bamboo US BidCo LLC (119087615)