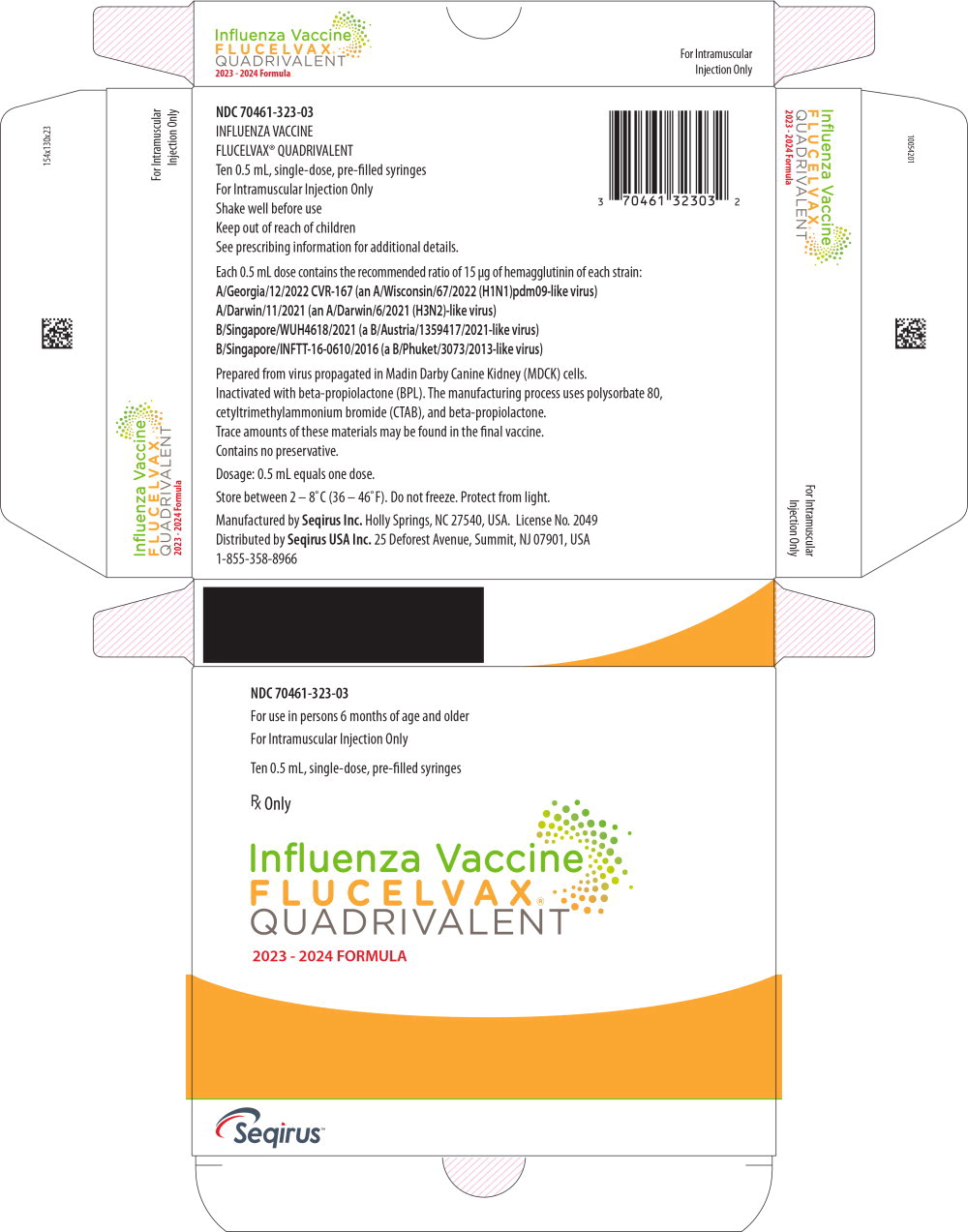
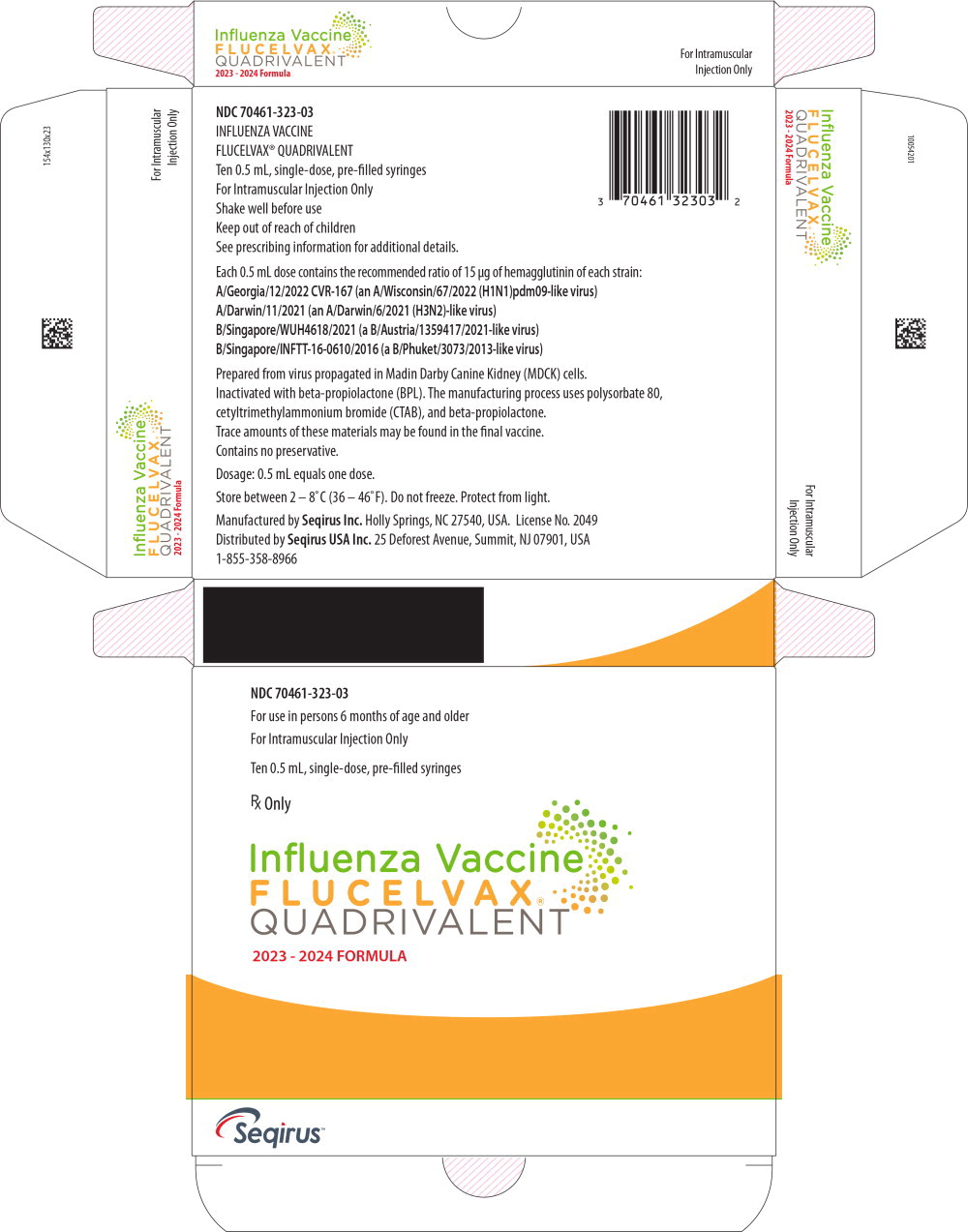
Label: FLUCELVAX QUADRIVALENT (influenza a virus a/georgia/12/2022 cvr-167 (h1n1) antigen (mdck cell derived, propiolactone inactivated), influenza a virus a/darwin/11/2021 (h3n2) antigen (mdck cell derived, propiolactone inactivated),influenza b virus b/singapore/wuh4618/2021 antigen (mdck cell derived, propiolactone inactivated),influenza b virus b/singapore/inftt-16-0610/2016 antigen- mdck cell derived, propiolactone inactivated injection, suspension
- NDC Code(s): 83703-037-03, 83703-037-04
- Packager: Bamboo US BidCo LLC
- Category: VACCINE LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated June 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FLUCELVAX QUADRIVALENT
influenza a virus a/georgia/12/2022 cvr-167 (h1n1) antigen (mdck cell derived, propiolactone inactivated), influenza a virus a/darwin/11/2021 (h3n2) antigen (mdck cell derived, propiolactone inactivated),influenza b virus b/singapore/wuh4618/2021 antigen (mdck cell derived, propiolactone inactivated),influenza b virus b/singapore/inftt-16-0610/2016 antigen (mdck cell derived, propiolactone inactivated) injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:83703-037 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INFLUENZA A VIRUS A/GEORGIA/12/2022 CVR-167 (H1N1) ANTIGEN (MDCK CELL DERIVED, PROPIOLACTONE INACTIVATED) (UNII: B8P3XN76W4) (INFLUENZA A VIRUS A/GEORGIA/12/2022 CVR-167 (H1N1) HEMAGGLUTININ ANTIGEN (MDCK CELL DERIVED, PROPIOLACTONE INACTIVATED) - UNII:N8JKS3VK2A) INFLUENZA A VIRUS A/GEORGIA/12/2022 CVR-167 (H1N1) HEMAGGLUTININ ANTIGEN (MDCK CELL DERIVED, PROPIOLACTONE INACTIVATED) 15 ug in 0.5 mL INFLUENZA A VIRUS A/DARWIN/11/2021 (H3N2) ANTIGEN (MDCK CELL DERIVED, PROPIOLACTONE INACTIVATED) (UNII: PV9K2R8HRM) (INFLUENZA A VIRUS A/DARWIN/11/2021 (H3N2) HEMAGGLUTININ ANTIGEN (MDCK CELL DERIVED, PROPIOLACTONE INACTIVATED) - UNII:C6PM4NXJ33) INFLUENZA A VIRUS A/DARWIN/11/2021 (H3N2) HEMAGGLUTININ ANTIGEN (MDCK CELL DERIVED, PROPIOLACTONE INACTIVATED) 15 ug in 0.5 mL INFLUENZA B VIRUS B/SINGAPORE/WUH4618/2021 ANTIGEN (MDCK CELL DERIVED, PROPIOLACTONE INACTIVATED) (UNII: TUE3AGP9ME) (INFLUENZA B VIRUS B/SINGAPORE/WUH4618/2021 HEMAGGLUTININ ANTIGEN (MDCK CELL DERIVED, PROPIOLACTONE INACTIVATED) - UNII:C76JLA4DM6) INFLUENZA B VIRUS B/SINGAPORE/WUH4618/2021 HEMAGGLUTININ ANTIGEN (MDCK CELL DERIVED, PROPIOLACTONE INACTIVATED) 15 ug in 0.5 mL INFLUENZA B VIRUS B/SINGAPORE/INFTT-16-0610/2016 ANTIGEN (MDCK CELL DERIVED, PROPIOLACTONE INACTIVATED) (UNII: G4VX46P9M6) (INFLUENZA B VIRUS B/SINGAPORE/INFTT-16-0610/2016 HEMAGGLUTININ ANTIGEN (MDCK CELL DERIVED, PROPIOLACTONE INACTIVATED) - UNII:0JXM8X6ZW8) INFLUENZA B VIRUS B/SINGAPORE/INFTT-16-0610/2016 HEMAGGLUTININ ANTIGEN (MDCK CELL DERIVED, PROPIOLACTONE INACTIVATED) 15 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength POTASSIUM CHLORIDE (UNII: 660YQ98I10) SODIUM CHLORIDE (UNII: 451W47IQ8X) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: 94255I6E2T) POTASSIUM PHOSPHATE, MONOBASIC (UNII: 4J9FJ0HL51) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83703-037-03 10 in 1 CARTON 1 NDC:83703-037-04 0.5 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125408 07/01/2023 07/31/2024 Labeler - Bamboo US BidCo LLC (119087615) Establishment Name Address ID/FEI Business Operations Seqirus Inc. 080102141 manufacture, label, pack, analysis