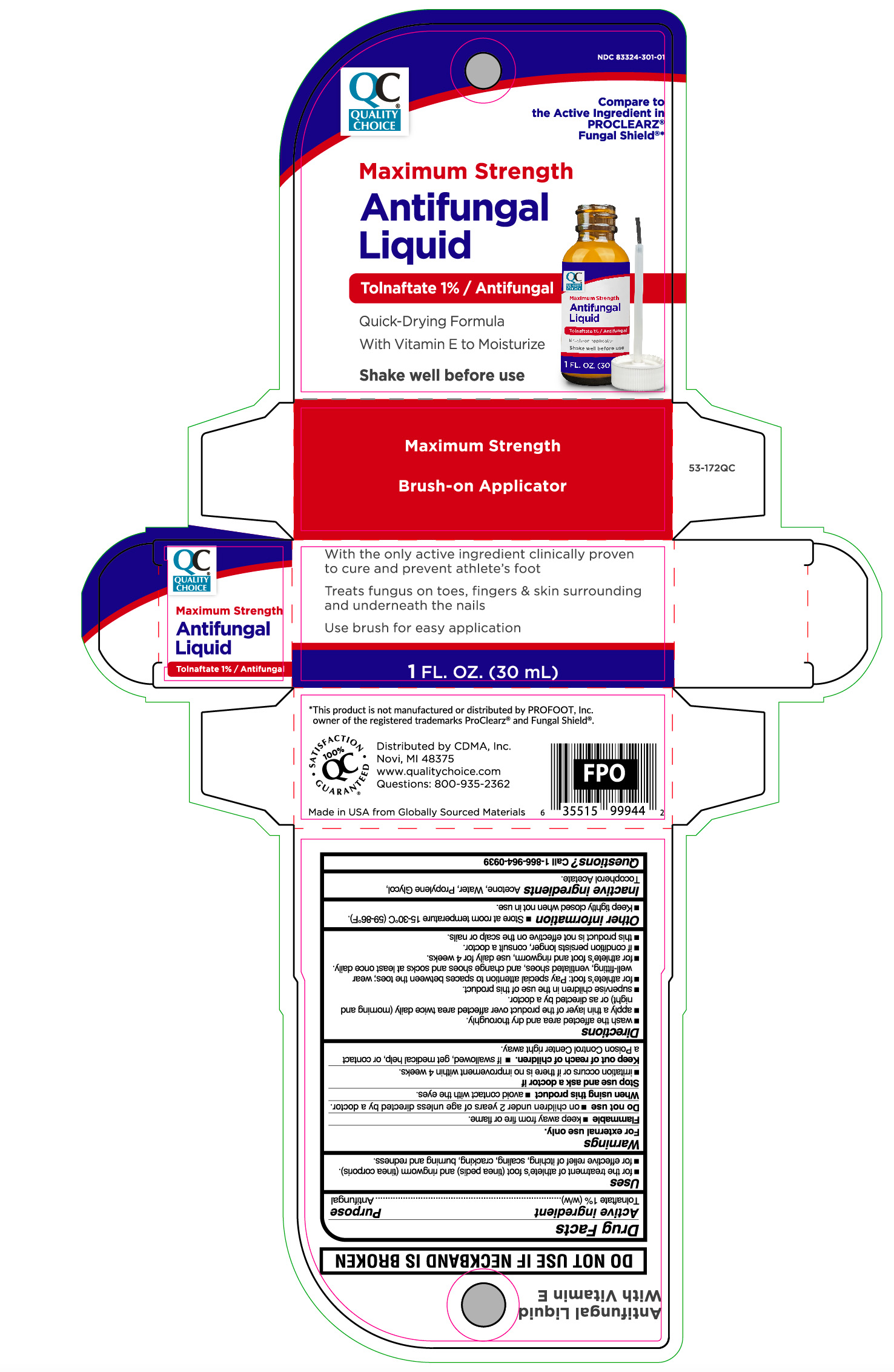
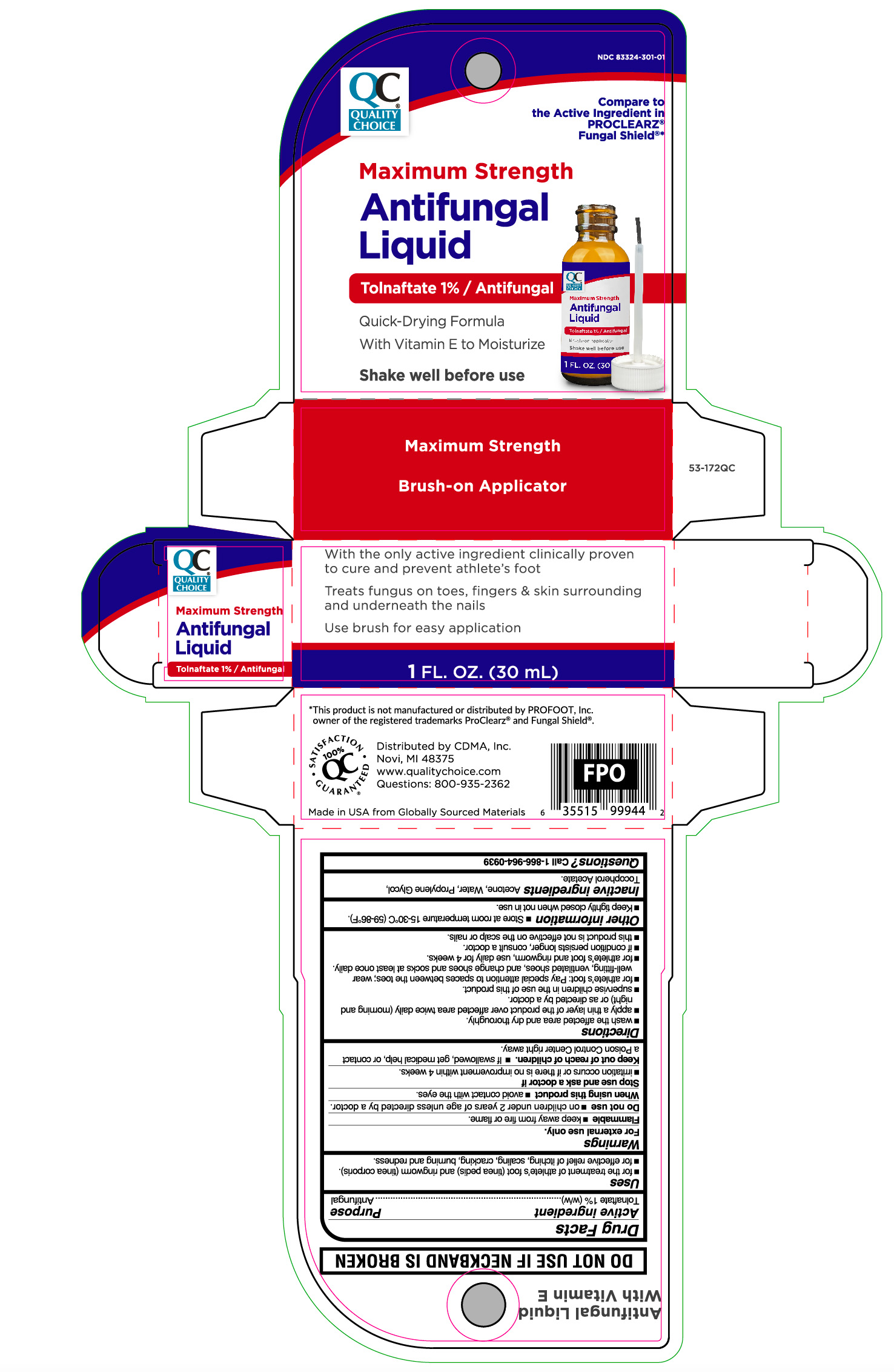
Label: MAXIMUM STRENGTH ANTIFUNGAL LIQUID- tolnaftate liquid
- NDC Code(s): 83324-301-01
- Packager: Chain Drug Marketing Association Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
- wash the affected area and dry thoroughly.
- apply a thin layer of the product over the affected area twice daily (morning and night) or as directed by a doctor.
- supervise children in the use of this product.
- for athlete's foot: pay special attention to spaces between toes; wear well-fitting, ventilated shoes, and change shoes and socks at least once daily.
- for athlete's foot and ringworm, use daily for 4 weeks.
- If condition persists longer, consult a doctor.
- this product is not effective on the scalp or nails.
- Other information
- Inactive ingredient
- Questions?
-
Principal Display Panel
QC
Quality
Choice
Maximum Strength
Antifungal
Liquid
TOLNAFTATE 1%/ Antifungal
Quick-Drying Formula
With Vitamin E to Moisturize
Shake well before use
Maximum Strength
Brush-on Applicator
With the only active ingredient clinically proven
to cure and prevent athlete's foot
Treats fungus on toes, fingers & skin surrounding
and underneath the nails
Use brush for easy application
1 FL. OZ. (30 mL)

-
INGREDIENTS AND APPEARANCE
MAXIMUM STRENGTH ANTIFUNGAL LIQUID
tolnaftate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83324-301 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) TOCOPHEROL (UNII: R0ZB2556P8) ACETONE (UNII: 1364PS73AF) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83324-301-01 1 in 1 CARTON 06/07/2024 1 30 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 06/07/2024 Labeler - Chain Drug Marketing Association Inc (011920774)