Label: DUVYZAT- givinostat suspension
- NDC Code(s): 11797-110-01, 11797-110-02
- Packager: Italfarmaco SPA
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DUVYZAT safely and effectively. See full prescribing information for DUVYZAT. DUVYZAT (givinostat) oral suspension - Initial U.S ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEDUVYZAT is indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients 6 years of age and older.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Evaluation and Testing Before Initiation of DUVYZAT - Obtain and evaluate baseline platelet counts and triglycerides prior to initiation of DUVYZAT [see Warnings and Precautions ...
-
3 DOSAGE FORMS AND STRENGTHSOral suspension: 8.86 mg/mL givinostat as a white to off-white or faintly pink, peach-cream flavored suspension.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Hematological Changes - DUVYZAT can cause dose-related thrombocytopenia and other signs of myelosuppression, including decreased hemoglobin and neutropenia. In Study 1 [see Clinical Studies ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described below and elsewhere in the labeling: Hematological Changes [see Warnings and Precautions (5.1)] Increased Triglycerides [see ...
-
7 DRUG INTERACTIONS7.1 Effect of DUVYZAT on Other Drugs - CYP3A4 Sensitive Substrates - Givinostat is a weak intestinal CYP3A4 inhibitor [see Clinical Pharmacology (12.3)]. Closely monitor when DUVYZAT is used in ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - DUVYZAT is indicated for the treatment of DMD, which is a disease of predominantly young male patients. Therefore, there are no adequate data available to ...
-
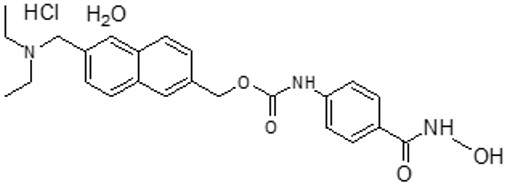
11 DESCRIPTIONDUVYZAT (givinostat) oral suspension contains givinostat hydrochloride monohydrate, a histone deacetylase inhibitor. Givinostat hydrochloride monohydrate is designated chemically as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - DUVYZAT is a histone deacetylase inhibitor. The precise mechanism by which DUVYZAT exerts its effect in patients with DMD is unknown. 12.2 ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Studies to assess the carcinogenic potential of givinostat have not been conducted. Mutagenesis - Givinostat was ...
-
14 CLINICAL STUDIESThe effectiveness of DUVYZAT for the treatment of Duchenne muscular dystrophy (DMD) was evaluated in a randomized, double-blind, placebo-controlled 18-month study (Study 1; NCT02851797). A total ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - DUVYZAT (givinostat) oral suspension is a white to off-white or faintly pink, peach-cream flavored suspension. It is supplied in an amber polyethylene terephthalate bottle ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use). Administration Instructions - Instruct patients or caregivers to [see Dosage and ...
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration.Issued: 3/2024 - MEDICATION GUIDE - DUVYZAT™ (doo' vi zat) (givinostat) oral suspension - What is the most ...
-
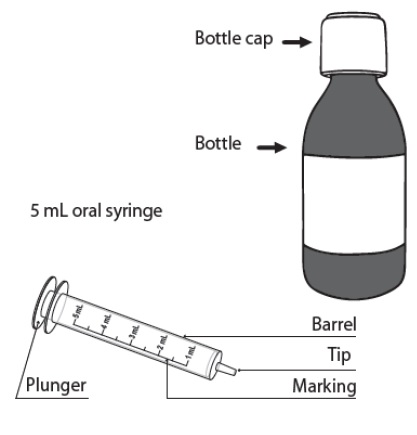
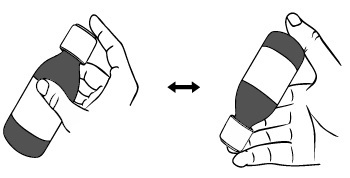
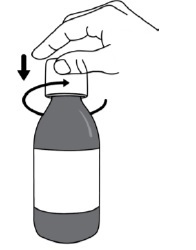
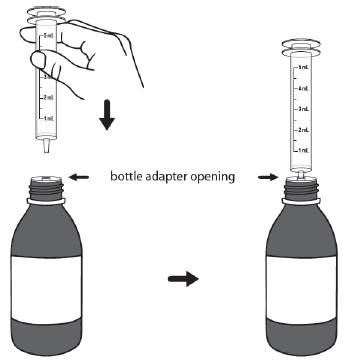
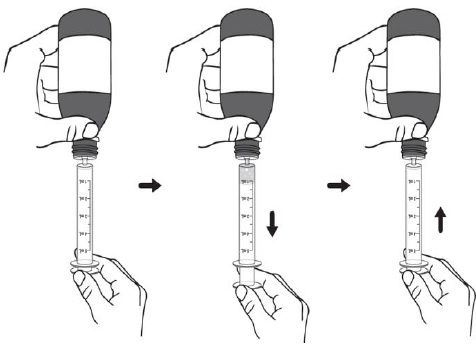
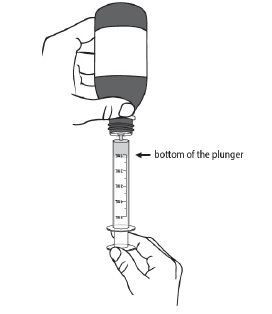
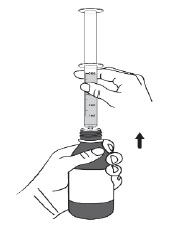
PATIENT PACKAGE INSERTINSTRUCTIONS FOR USE - DUVYZAT [doo' vi zat] (givinostat) oral suspension - This Instructions for Use contains information on how to take DUVYZAT. Ask your healthcare provider or pharmacist if ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Italfarmaco S.A. Madrid, Spain - Distributed by: ITF Therapeutics, LLC - Concord, MA 01742 - DUVYZAT is a trademark owned by Italfarmaco S.p.A - All rights reserved.
-
PRINCIPAL DISPLAY PANELNDC 11797-110-01 - Rx only - DuvyzatTM - [givinostat] Oral suspension - 8.86 mg/mL - For Oral Administration Only - Shake bottle for 30 seconds - before use - 140 mL - Dispense a ...
-
PRINCIPAL DISPLAY PANELNDC 11797-110-02 - Rx only - DuvyzatTM - [givinostat] Oral suspension - 8.86 mg/mL - For Oral - Administration Only - Shake bottle for 30 seconds - before use - See enclosed ...
-
INGREDIENTS AND APPEARANCEProduct Information