Label: VILAZODONE- vilazodone hydrochloride tablet
VILAZODONE- vilazodone hydrochloride tablet
- NDC Code(s): 42291-948-30, 42291-949-30, 42291-950-30
- Packager: AvKARE
- This is a repackaged label.
- Source NDC Code(s): 60505-4772, 60505-4773, 60505-4774
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated June 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONVilazodone Hydrochloride Tablets - Rx Only - These highlights do not include all the information needed to use - VILAZODONEsafely and effectively.See full prescribing information ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING:SUICIDAL THOUGHTS AND BEHAVIORS
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening and for emergence of suicidal thoughts and behaviors[see Warnings and Precautions (5.1)]. Vilazodone is not approved for use in pediatric patients[see Use in Specific Populations (8.4)].
Close -
1 INDICATIONS AND USAGE
Vilazodone is indicated for the treatment of major depressive disorder (MDD) in adults - [see Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosagefor Treatment ofMajor Depressive Disorder - The recommended target dosage for vilazodone is 20 mg to 40 mg orally once daily with food - [see Clinical Pharmacology (12.3) ...
-
3 DOSAGE FORMS AND STRENGTHS
Vilazodone tablets are available as 10 mg, 20 mg and 40 mg film-coated tablets. 10 mg pink, oval tablet, debossed with 10 on one side - 20 mg orange, oval tablet, debossed with 20 on one side ...
-
4 CONTRAINDICATIONS
Vilazodone is contraindicated in: Patients taking, or within 14 days of stopping, monoamine oxidase inhibitors (MAOIs), including MAOIs such as linezolid or intravenous methylene blue, because of ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Thoughts and Behavior in Adolescents and Young Adults - In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that ...
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling: Suicidal Thoughts and Behaviors in Adolescents and Young Adults - [see Warnings and ...
-
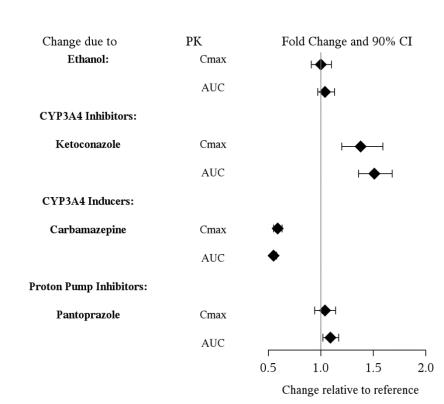
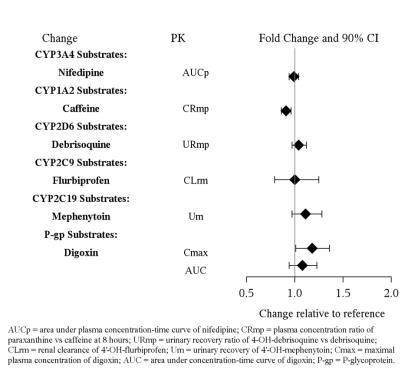
7 DRUG INTERACTIONS
7.1 Drugs Having Clinically Important Interactions WithVilazodone - Table 4: Clinically Important Drug Interactions with Vilazodone - Concomitant Drug Nameor Drug ClassClinical ...
-
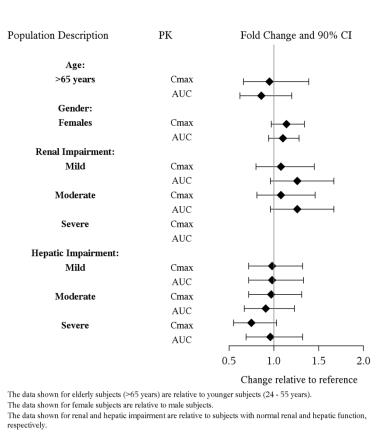
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antidepressants during pregnancy. Healthcare ...
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance - Vilazodone is not a controlled substance. 9.2 Abuse and Dependence - Vilazodone has been systematically studied in animals and did not demonstrate abuse ...
-
10 OVERDOSAGE
There is limited clinical trial experience regarding human overdose with vilazodone. The adverse reactions associated with overdose of vilazodone at doses of 200-280 mg (5 to 7 times the ...
-
11 DESCRIPTION
VILAZODONE tablets for oral administration contain polymorph Form IV vilazodone hydrochloride (HCl), a selective serotonin reuptake inhibitor and a 5HT - 1A receptor partial agonist ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of action - The mechanism of action of vilazodone in the treatment of major depressive disorder is not fully understood, but is thought to be related to its enhancement of ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Carcinogenicity studies were conducted in which B6C3F1mice and Wistar rats were given oral doses of ...
-
14 CLINICAL STUDIES
The efficacy of vilazodone as a treatment for major depressive disorder was demonstrated in four multicenter, randomized, double-blind, placebo-controlled studies in adult (18-70 years of age ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Vilazodone tablets are supplied in the following configurations: Tablet - StrengthTablet - Color/ShapeTablet - MarkingsPackage - ConfigurationNDC Code - 10 mgpink, oval tabletdebossed ...
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling ( Medication Guide). Suicidal Thoughts and Behaviors - Advise patients and caregivers to look for the emergence of suicidality ...
-
MEDICATION GUIDEMEDICATION GUIDE - Vilazodone - Tablets, for oral use - What is the most important information I should know aboutVilazodone? Vilazodonemay cause serious side effects ...
-
PRINCIPAL DISPLAY PANEL

-
PRINCIPAL DISPLAY PANEL

-
PRINCIPAL DISPLAY PANEL

-
INGREDIENTS AND APPEARANCEProduct Information