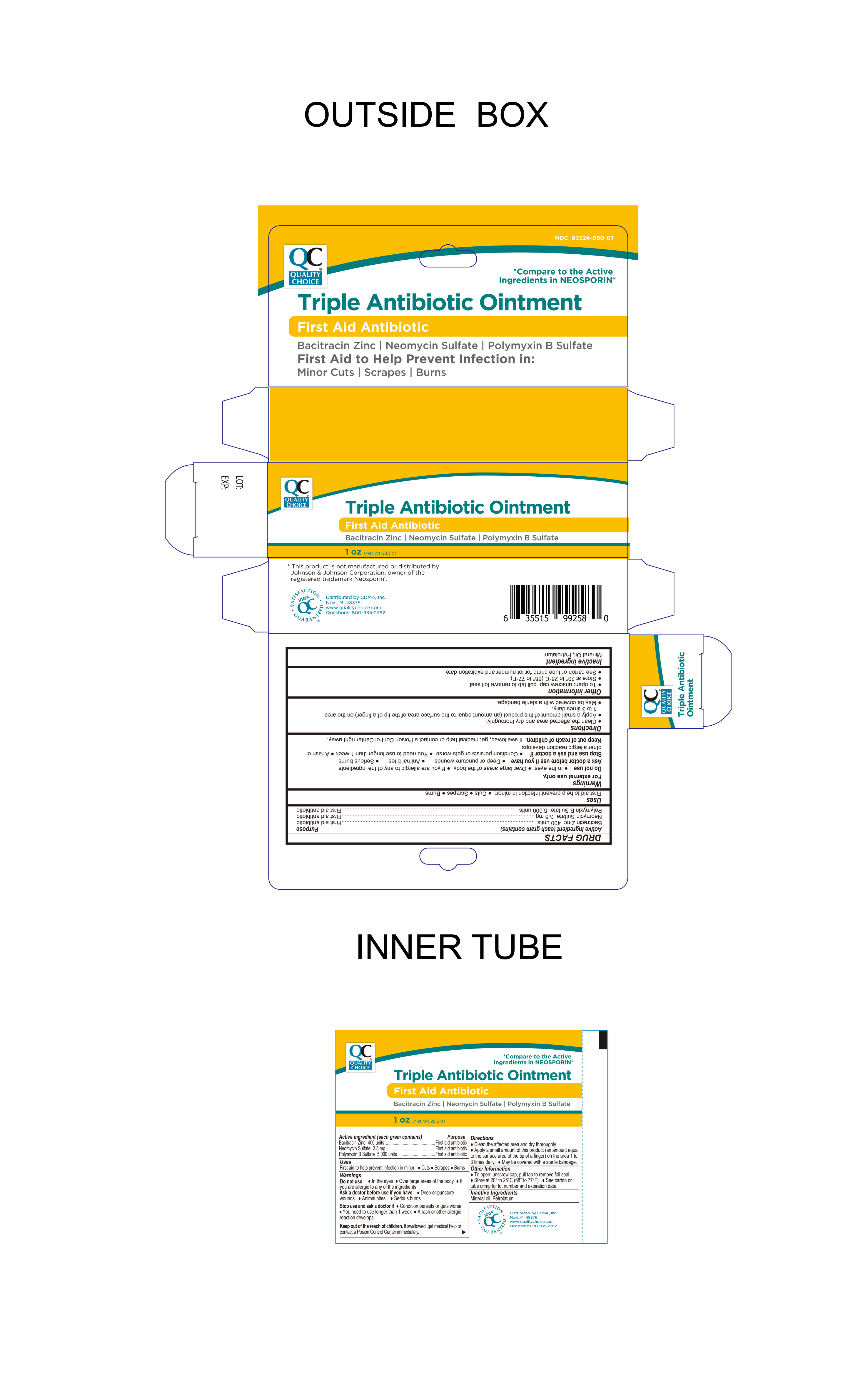
Label: QC TRIPLE ANTIBIOTIC- bacitracin zinc, neomycin sulfate , polymyxin b sulfate. ointment
- NDC Code(s): 83324-050-01, 83324-050-05
- Packager: Chain Drug Marketing Association
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
-
Active ingredients (each gram contains)
Bacitracin zinc 400 units - Neomycin sulfate 3.5 mg - Polymyxin B sulfate 5,000 units
-
Purpose
First Aid Antibiotic Ointment
-
INDICATIONS & USAGEUses first aid to help prevent infection in minor:● cuts ● scrapes ● burns
-
WarningsFor external use only.
-
Do not use
In the eyes - Over large areas of the body - If you are allergic to any of the ingredients
-
Stop Use and ask a doctor if
Condition persists or gets worse - You need to use longer than 1 week - A rash or other allergic reaction develops
-
Ask Doctor before use if you have
Deep or puncture wounds - Animal bites - Serious burns
-
KEEP OUT OF REACH OF CHILDRENKeep out of reach of children.If swallowed, get medical help or contact a Poison Control Center immediately.
-
Directions
● clean the affected area and dry thoroughly. ● apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily - ● may be covered ...
-
Other information
● To open: unscrew cap, pull tab to remove foil seal - ● store at 20° to 25°C ( 68° to 77°F) ● see carton or tube crimp for lot number and expiration date.
-
INACTIVE INGREDIENTInactive ingredientMineral Oil, Petrolatum
-
SPL UNCLASSIFIED SECTIONDistributed by CDMA., Inc. © 43157 W 9 Mile Rd. Novi, MI. 48375 - www.qualitychoice.com - Questions: 800-935-2362
-
Other Information:
This product is not manufactured or distributed by - Johnson & Johnson Corporation, owner of the registered trademark Neosporin®
-
Packaging

-
PRINCIPAL DISPLAY PANEL
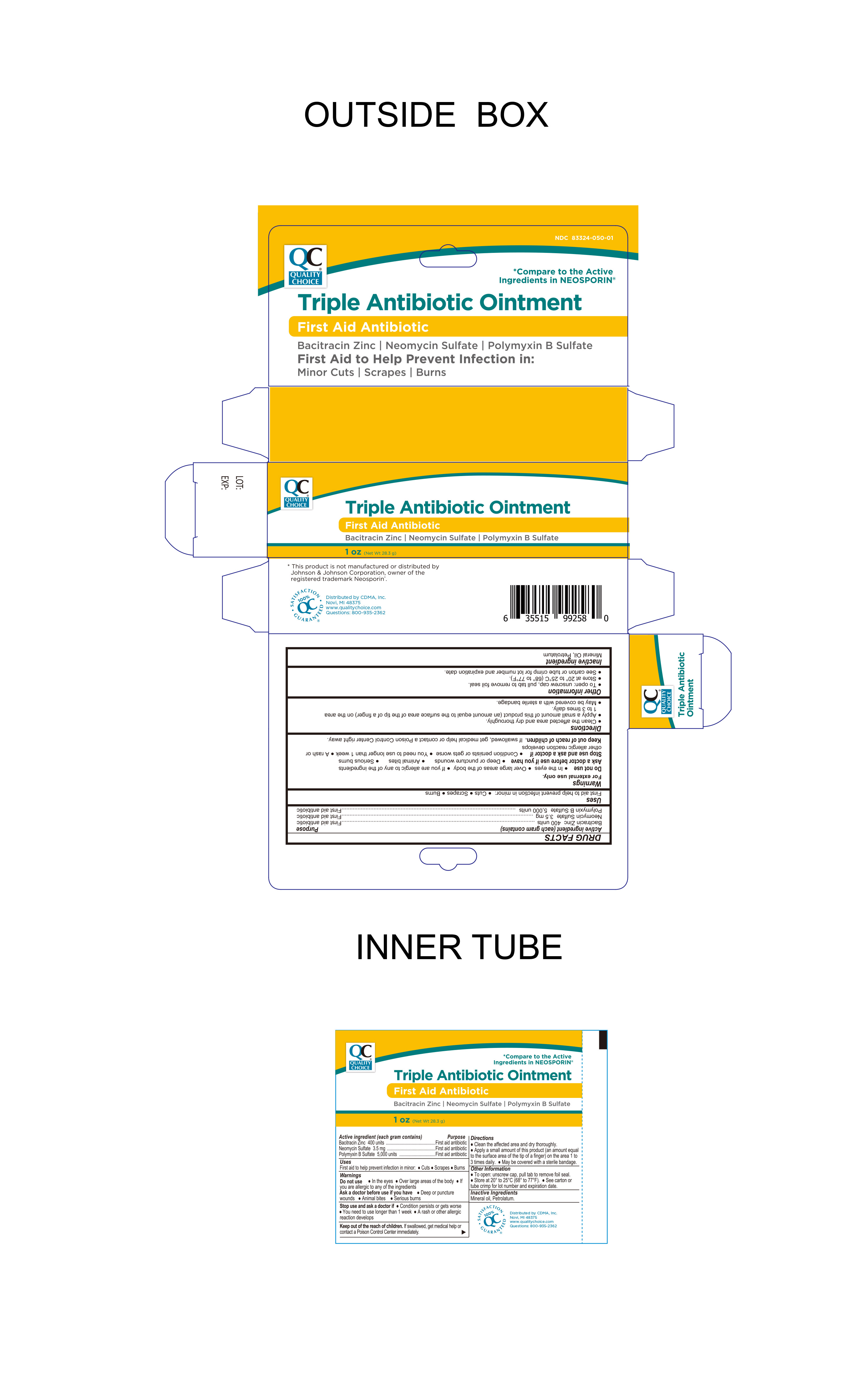
-
INGREDIENTS AND APPEARANCEProduct Information