Label: LUBIPROSTONE capsule
- NDC Code(s): 43602-548-05, 43602-548-30, 43602-549-05, 43602-549-30
- Packager: Ascent Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LUBIPROSTONE CAPSULES safely and effectively. See full prescribing information for LUBIPROSTONE CAPSULES. LUBIPROSTONE capsules ...
-
Table of ContentsTable of Contents
-
1. INDICATIONS AND USAGE1.1 Chronic Idiopathic Constipation in Adults - Lubiprostone capsules are indicated for the treatment of chronic idiopathic constipation (CIC) in adults. 1.2 Opioid-Induced Constipation ...
-
2. DOSAGE AND ADMINISTRATION
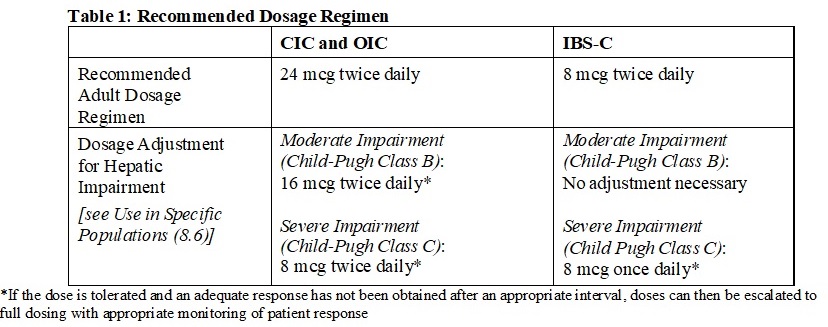
2.1 Recommended Dosage - The recommended oral dosage of lubiprostone capsules by indication and adjustments for patients with moderate (Child Pugh Class B) and severe (Child Pugh Class C ...
-
3. DOSAGE FORMS AND STRENGTHS
Lubiprostone capsules is available as an oval, gelatin capsule containing 8 mcg or 24 mcg of lubiprostone. 8 mcg capsules are light orange oval capsules containing clear liquid printed with '548 ...
-
4. CONTRAINDICATIONS
Lubiprostone capsules are contraindicated in patients with known or suspected mechanical gastrointestinal obstruction [see Warnings and Precautions (5.5)].
-
5. WARNINGS AND PRECAUTIONS
5.1 Nausea - Patients taking lubiprostone capsules may experience nausea. Concomitant administration of food with lubiprostone capsules may reduce symptoms of nausea [see Adverse Reactions ...
-
6 ADVERSE REACTIONS
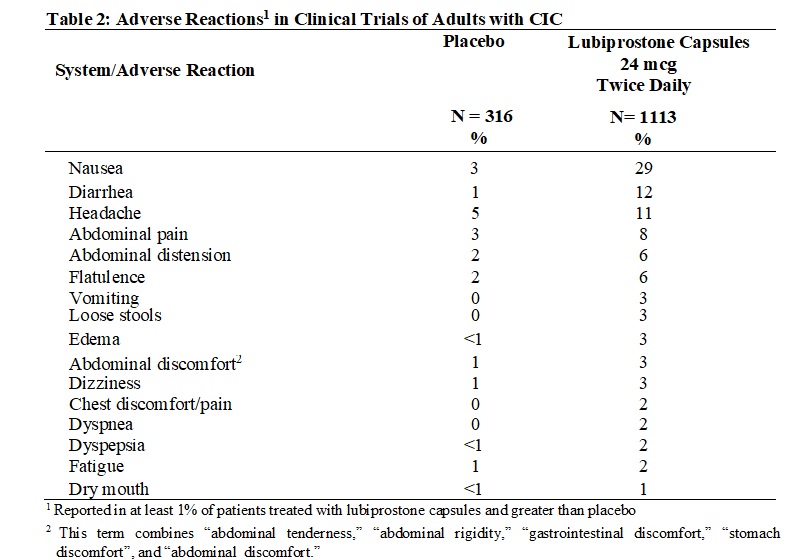
The following adverse reactions are described below and elsewhere in labeling: Nausea [see Warnings and Precautions (5.1)] Diarrhea [see Warnings and Precautions (5.2)] Syncope and Hypotension ...
-
7 DRUG INTERACTIONS
7.1 Methadone - Diphenylheptane opioids (e.g., methadone) have been shown in nonclinical studies to dose-dependently reduce the activation of ClC-2 by lubiprostone in the gastrointestinal ...
-
8. USE IN SPECIFIC POPULATIONS
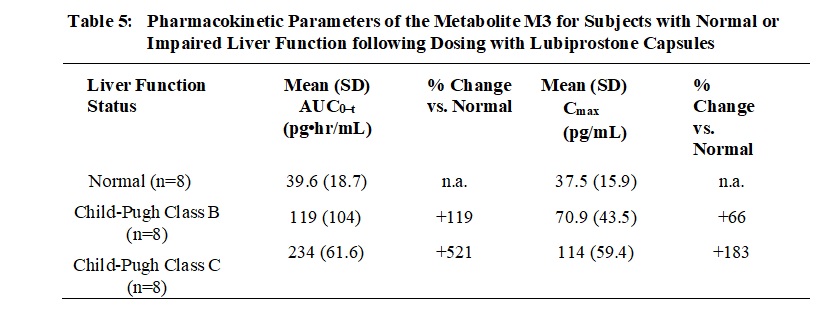
8.1 Pregnancy - Risk Summary - Following oral administration, concentrations of lubiprostone in plasma are below the level of quantitation; however, one of the metabolites, M3, has measurable ...
-
10. OVERDOSAGE
There have been six reports of overdosage with lubiprostone capsules during clinical development. Of these six cases, only two subjects reported adverse events: one reported vomiting, diarrhea and ...
-
11. DESCRIPTION
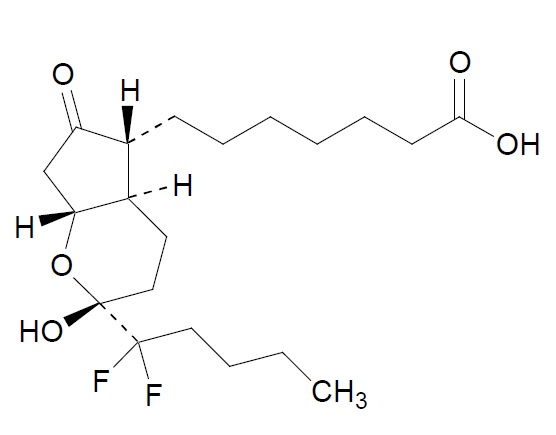
Lubiprostone is a chloride channel activator for oral use. The chemical name for lubiprostone is ...
-
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Lubiprostone is a locally acting chloride channel activator that enhances a chloride-rich intestinal fluid secretion without altering sodium and potassium ...
-
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Two 2-year oral (gavage) carcinogenicity studies (one in Crl:B6C3F1 mice and one in Sprague-Dawley rats) were ...
-
14. CLINICAL STUDIES
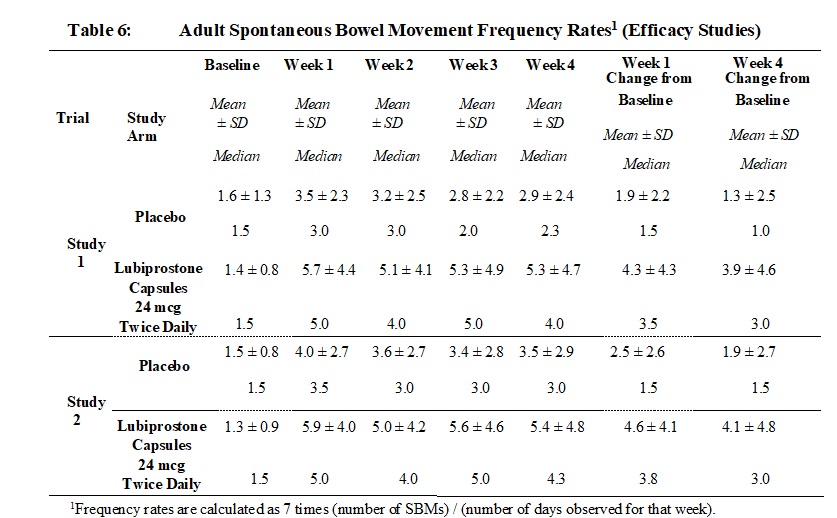
14.1 Chronic Idiopathic Constipation in Adults - Two double-blinded, placebo-controlled studies of identical design were conducted in patients with CIC. CIC was defined as, on average, less ...
-
16. HOW SUPPLIED/STORAGE AND HANDLING
Lubiprostone capsules are available as: The 8 mcg lubiprostone capsules light orange oval capsules containing clear liquid printed with '548' with black ink. Bottles of 30 NDC ...
-
17. PATIENT COUNSELING INFORMATION
Administration Instructions - Instruct patients to take lubiprostone capsules orally with food and water to reduce the occurrence of nausea [see Warnings and Precautions (5.1)]. Swallow capsules ...
-
PRINCIPAL DISPLAY PANELLubiprostone Capsules 8 mcg 500s count - Lubiprostone Capsules 24 mcg 500s count
-
INGREDIENTS AND APPEARANCEProduct Information