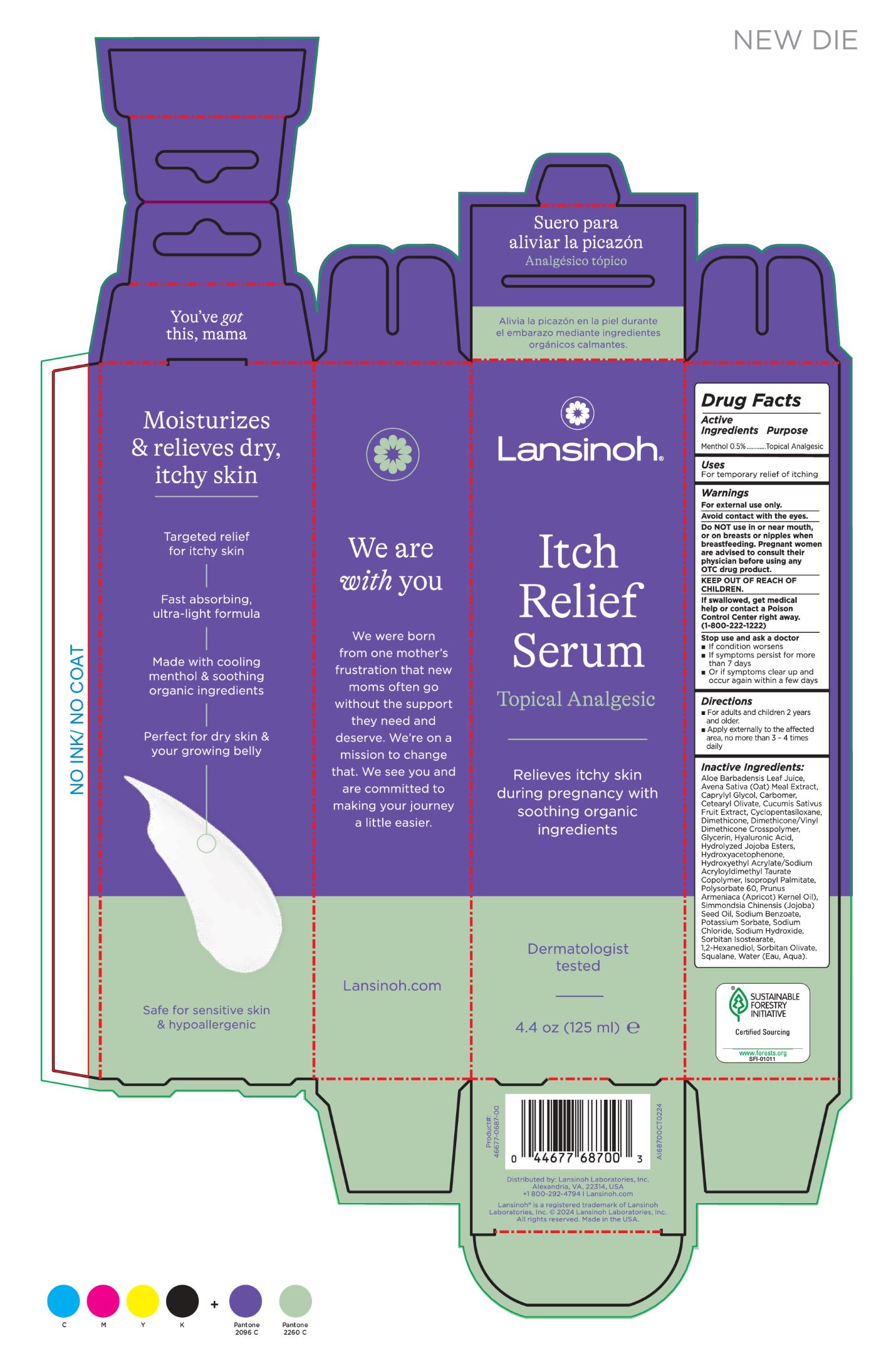
Label: LANSINOH ANTI-ITCH- menthol 0.5% cream
- NDC Code(s): 53997-002-01, 53997-002-02
- Packager: Lansinoh Laboratories Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- DO NOT USE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- QUESTIONS
- STOP USE
- WARNINGS
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive Ingredients:
Aloe Barbadensis Leaf Juice, Avena Sativa (Oat) Meal Extract, Caprylyl Glycol, Carbomer, Cetearyl Olivate, Cucumis Sativus Fruit Extract, Cyclopentasiloxane, Dimethicone, Dimethicone/Vinyl Dimethicone Crosspolymer, Glycerin, Hyaluronic Acid, Hydrolyzed Jojoba Esters, Hydroxyacetophenone, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Isopropyl Palmitate, Polysorbate 60, Prunus Armeniaca (Apricot) Kernel Oil), Simmondsia Chinensis (Jojoba) Seed Oil, Sodium Benzoate, Potassium Sorbate, Sodium Chloride, Sodium Hydroxide, Sorbitan Isostearate, 1,2-Hexanediol, Sorbitan Olivate, Squalane, Water (Eau, Aqua). - INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LANSINOH ANTI-ITCH
menthol 0.5% creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53997-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.5 g in 100 mL Inactive Ingredients Ingredient Name Strength CUCUMBER (UNII: YY7C30VXJT) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM HYDROXIDE (UNII: 55X04QC32I) DIMETHICONE (UNII: 92RU3N3Y1O) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) GLYCERIN (UNII: PDC6A3C0OX) SQUALANE (UNII: GW89575KF9) JOJOBA OIL (UNII: 724GKU717M) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) POLYSORBATE 60 (UNII: CAL22UVI4M) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) HYALURONIC ACID (UNII: S270N0TRQY) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) CETEARYL OLIVATE (UNII: 58B69Q84JO) WATER (UNII: 059QF0KO0R) HYDROLYZED JOJOBA ESTERS (POTASSIUM SALTS) (UNII: CH428W5O62) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) SODIUM CHLORIDE (UNII: 451W47IQ8X) SORBITAN OLIVATE (UNII: MDL271E3GR) APRICOT KERNEL OIL (UNII: 54JB35T06A) SODIUM BENZOATE (UNII: OJ245FE5EU) OATMEAL (UNII: 8PI54V663Y) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (HARD PARTICLE) (UNII: H895X08VNQ) ALOE VERA LEAF (UNII: ZY81Z83H0X) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53997-002-02 1 in 1 CARTON 05/15/2024 1 NDC:53997-002-01 125 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 05/15/2024 Labeler - Lansinoh Laboratories Inc (181696907) Establishment Name Address ID/FEI Business Operations Dhaliwal Pharmaceuticals Laboratories, LLC 116933772 manufacture(53997-002)