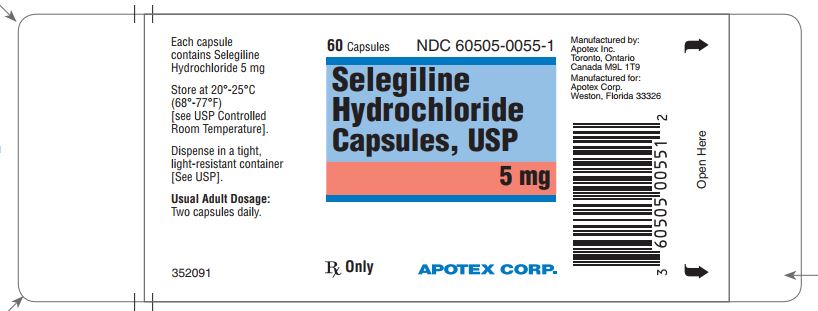
Label: SELEGILINE HYDROCHLORIDE capsule
- NDC Code(s): 60505-0055-1, 60505-0055-2, 60505-0055-3
- Packager: Apotex Corp.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
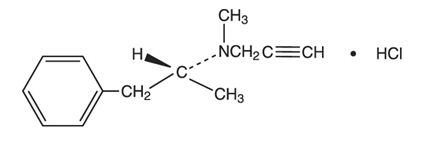
Selegiline hydrochloride is a levorotatory acetylenic derivative of phenethylamine. It is commonly referred to in the clinical and pharmacological literature as l- deprenyl.
The chemical name is: (R)-(-)-N,α-Dimethyl-N-2-propynylphenethylamine hydrochloride. It is a white to near white crystalline powder, freely soluble in water, chloroform, and methanol, and has a molecular weight of 223.75. The molecular formula is C13H17N.HCI and the structural formula is as follows:
Each capsule, for oral administration contains 5 mg of selegiline hydrochloride. In addition, each capsule contains the following inactive ingredients: Anhydrous Lactose NF, Citric Acid Anhydrous USP, Microcrystalline Cellulose NF PH102, Stearic Acid NF and Talc USP. The capsule shell contains Gelatin NF, FD & C Blue #1 and Titanium Dioxide. The capsule logo ink Black SW-9008/SW-9009 contains the following inactive ingredients: Ammonium Hydroxide; Black Iron Oxide, Bacteria Controlled EEC No. 172; n-Butyl NF; Ethyl Alcohol, Anhydrous, 200 Proof; Isopropyl Alcohol USP; Potassium Hydroxide NF; Propylene Glycol USP; Purified Water USP and Shellac NF.
-
CLINICAL PHARMACOLOGY
The mechanisms accounting for selegiline's beneficial adjunctive action in the treatment of Parkinson's disease are not fully understood. Inhibition of monoamine oxidase, type B, activity is generally considered to be of primary importance; in addition, there is evidence that selegiline may act through other mechanisms to increase dopaminergic activity.
Selegiline is best known as an irreversible inhibitor of monoamine oxidase (MAO), an intracellular enzyme associated with the outer membrane of mitochondria. Selegiline inhibits MAO by acting as a 'suicide' substrate for the enzyme; that is, it is converted by MAO to an active moiety which combines irreversibly with the active site and/or the enzyme's essential FAD cofactor. Because selegiline has greater affinity for type B rather than for type A active sites, it can serve as a selective inhibitor of MAO type B if it is administered at the recommended dose.
MAOs are widely distributed throughout the body; their concentration is especially high in liver, kidney, stomach, intestinal wall, and brain. MAOs are currently subclassified into two types, A and B, which differ in their substrate specificity and tissue distribution. In humans, intestinal MAO is predominantly type A, while most of that in brain is type B.
In CNS neurons, MAO plays an important role in the catabolism of catecholamines (dopamine, norepinephrine and epinephrine) and serotonin. MAOs are also important in the catabolism of various exogenous amines found in a variety of foods and drugs. MAO in the GI tract and liver (primarily type A), for example, is thought to provide vital protection from exogenous amines (e.g., tyramine) that have the capacity, if absorbed intact, to cause a 'hypertensive crisis,' the so-called 'cheese reaction.' (If large amounts of certain exogenous amines gain access to the systemic circulation - e.g., from fermented cheese, red wine, herring, over-the-counter cough/cold medications, etc. - they are taken up by adrenergic neurons and displace norepinephrine from storage sites within membrane bound vesicles. Subsequent release of the displaced norepinephrine causes the rise in systemic blood pressure, etc.)
In theory, since MAO A of the gut is not inhibited, patients treated with selegiline at a dose of 10 mg a day should be able to take medications containing pharmacologically active amines and consume tyramine-containing foods without risk of uncontrolled hypertension. Although rare, a few reports of hypertensive reactions have occurred in patients receiving selegiline at the recommended dose, with tyramine-containing foods. In addition, one case of hypertensive crisis has been reported in a patient taking the recommended dose of selegiline and a sympathomimetic medication, ephedrine. The pathophysiology of the 'cheese reaction' is complicated and, in addition to its ability to inhibit MAO B selectively, selegiline's relative freedom from this reaction has been attributed to an ability to prevent tyramine and other indirect acting sympathomimetics from displacing norepinephrine from adrenergic neurons. However, until the pathophysiology of the ‘cheese reaction’ is more completely understood, it seems prudent to assume that selegiline can ordinarily only be used safely without dietary restrictions at doses where it presumably selectively inhibits MAO B (e.g., 10 mg/day).
In short, attention to the dose dependent nature of selegiline's selectivity is critical if it is to be used without elaborate restrictions being placed on diet and concomitant drug use although, as noted above, a few cases of hypertensive reactions have been reported at the recommended dose. (See WARNINGS and PRECAUTIONS.)
It is important to be aware that selegiline may have pharmacological effects unrelated to MAO B inhibition. As noted above, there is some evidence that it may increase dopaminergic activity by other mechanisms, including interfering with dopamine re-uptake at the synapse. Effects resulting from selegiline administration may also be mediated through its metabolites. Two of its three principal metabolites, amphetamine and methamphetamine, have pharmacological actions of their own; they interfere with neuronal uptake and enhance release of several neurotransmitters (e.g., norepinephrine, dopamine, serotonin). However, the extent to which these metabolites contribute to the effects of selegiline are unknown.
Rationale for the use of a Selective Monoamine Oxidase Type B Inhibitor in Parkinson's Disease
Many of the prominent symptoms of Parkinson's disease are due to a deficiency of striatal dopamine that is the consequence of a progressive degeneration and loss of a population of dopaminergic neurons which originate in the substantia nigra of the midbrain and project to the basal ganglia or striatum. Early in the course of Parkinson's Disease, the deficit in the capacity of these neurons to synthesize dopamine can be overcome by administration of exogenous levodopa, usually given in combination with a peripheral decarboxylase inhibitor (carbidopa).
With the passage of time, due to the progression of the disease and/or the effect of sustained treatment, the efficacy and quality of the therapeutic response to levodopa diminishes. Thus, after several years of levodopa treatment, the response, for a given dose of levodopa, is shorter, has less predictable onset and offset (i.e., there is `wearing off'), and is often accompanied by side effects (e.g., dyskinesia, akinesias, on-off phenomena, freezing, etc.).
This deteriorating response is currently interpreted as a manifestation of the inability of the ever decreasing population of intact nigrostriatal neurons to synthesize and release adequate amounts of dopamine.
MAO B inhibition may be useful in this setting because, by blocking the catabolism of dopamine, it would increase the net amount of dopamine available (i.e., it would increase the pool of dopamine). Whether or not this mechanism or an alternative one actually accounts for the observed beneficial effects of adjunctive selegiline is unknown.
Selegiline's benefit in Parkinson's disease has only been documented as an adjunct to levodopa/carbidopa. Whether or not it might be effective as a sole treatment is unknown, but past attempts to treat Parkinson's disease with non-selective MAOI monotherapy are reported to have been unsuccessful. It is important to note that attempts to treat Parkinsonian patients with combinations of levodopa and currently marketed non-selective MAO inhibitors were abandoned because of multiple side effects including hypertension, increase in involuntary movement, and toxic delirium.
Pharmacokinetic Information (Absorption, Distribution, Metabolism and Elimination-ADME)
The absolute bioavailability of selegiline following oral dosing is not known; however, selegiline undergoes extensive metabolism (presumably attributable to presystemic clearance in gut and liver). The major plasma metabolites are N-desmethylselegiline, L-amphetamine and L-methamphetamine. Only N-desmethylselegiline has MAO-B inhibiting activity. The peak plasma levels of these metabolites following a single oral dose of 10 mg are from 4 to almost 20 times greater than that of the maximum plasma concentration of selegiline [1 ng/mL]. The maximum concentrations of amphetamine and methamphetamine, however, are far below those ordinarily expected to produce clinically important effects.
Single oral dose studies do not predict multiple dose kinetics, however, at steady state the peak plasma level of selegiline is 4 fold that obtained following a single dose. Metabolite concentrations increase to a lesser extent, averaging 2 fold that seen after a single dose.
The bioavailability of selegiline is increased 3 to 4 fold when it is taken with food.
The extent of systemic exposure to selegiline at a given dose varies considerably among individuals. Estimates of systemic clearance of selegiline are not available. Following a single oral dose, the mean elimination half-life of selegiline is two hours. Under steady state conditions the elimination half-life increases to ten hours.
Because selegiline's inhibition of MAO-B is irreversible, it is impossible to predict the extent of MAO-B inhibition from steady state plasma levels. For the same reason, it is not possible to predict the rate of recovery of MAO-B activity as a function of plasma levels. The recovery of MAO-B activity is a function of de novo protein synthesis; however, information about the rate of de novo protein synthesis is not yet available. Although platelet MAO-B activity returns to the normal range within 5 to 7 days of selegiline discontinuation, the linkage between platelet and brain MAO-B inhibition is not fully understood nor is the relationship of MAO-B inhibition to the clinical effect established (see CLINICAL PHARMACOLOGY).
Renal Impairment
No pharmacokinetic information is available on selegiline or its metabolites in renally impaired subjects.
Hepatic Impairment
No pharmacokinetic information is available on selegiline or its metabolites in hepatically impaired subjects.
Age
Although a general conclusion about the effects of age on the pharmacokinetics of selegiline is not warranted because of the size of the sample evaluated (12 subjects greater than 60 years of age, 12 subjects between the ages of 18 to 30), systemic exposure was about twice as great in older as compared to a younger population given a single oral dose of 10 mg.
-
INDICATIONS AND USAGE
Selegiline capsules, USP are indicated as an adjunct in the management of Parkinsonian patients being treated with levodopa/carbidopa who exhibit deterioration in the quality of their response to this therapy. There is no evidence from controlled studies that selegiline has any beneficial effect in the absence of concurrent levodopa therapy.
Evidence supporting this claim was obtained in randomized controlled clinical investigations that compared the effects of added selegiline or placebo in patients receiving levodopa/carbidopa. Selegiline was significantly superior to placebo on all three principal outcome measures employed: change from baseline in daily levodopa/carbidopa dose, the amount of 'off' time, and patient self-rating of treatment success. Beneficial effects were also observed on other measures of treatment success (e.g., measures of reduced end of dose akinesia, decreased tremor and sialorrhea, improved speech and dressing ability and improved overall disability as assessed by walking and comparison to previous state).
-
CONTRAINDICATIONS
Selegiline is contraindicated in patients with a known hypersensitivity to this drug.
Selegiline is contraindicated for use with meperidine (DEMEROL & other trade names). This contraindication is often extended to other opioids. (See Drug Interactions.)
-
WARNINGS
Selegiline should not be used at daily doses exceeding those recommended (10 mg/day) because of the risks associated with non-selective inhibition of MAO. (SeeCLINICAL PHARMACOLOGY.)
The selectivity of selegiline for MAO B may not be absolute even at the recommended daily dose of 10 mg a day. Rare cases of hypertensive reactions associated with ingestion of tyramine-containing foods have been reported in patients taking the recommended daily dose of selegiline. The selectivity is further diminished with increasing daily doses. The precise dose at which selegiline becomes a non-selective inhibitor of all MAO is unknown, but may be in the range of 30 to 40 mg a day.
Severe CNS toxicity associated with hyperpyrexia and death have been reported with the combination of tricyclic antidepressants and non-selective MAOIs (Phenelzine, Tranylcypromine). A similar reaction has been reported for a patient on amitriptyline and selegiline. Another patient receiving protriptyline and selegiline developed tremors, agitation, and restlessness followed by unresponsiveness and death two weeks after selegiline was added. Related adverse events including hypertension, syncope, asystole, diaphoresis, seizures, changes in behavioral and mental status, and muscular rigidity have also been reported in some patients receiving selegiline and various tricyclic antidepressants.
Serious, sometimes fatal, reactions with signs and symptoms that may include hyperthermia, rigidity, myoclonus, autonomic instability with rapid fluctuations of the vital signs, and mental status changes that include extreme agitation progressing to delirium and coma have been reported with patients receiving a combination of fluoxetine hydrochloride and non- selective MAOIs. Similar signs have been reported in some patients on the combination of selegiline (10 mg a day) and selective serotonin reuptake inhibitors including fluoxetine, sertraline and paroxetine.
Since the mechanisms of these reactions are not fully understood, it seems prudent, in general, to avoid this combination of selegiline and tricyclic antidepressants as well as selegiline and selective serotonin reuptake inhibitors. At least 14 days should elapse between discontinuation of selegiline and initiation of treatment with a tricyclic antidepressant or selective serotonin reuptake inhibitors. Because of the long half-lives of fluoxetine and its active metabolite, at least five weeks (perhaps longer, especially if fluoxetine has been prescribed chronically and/or at higher doses) should elapse between discontinuation of fluoxetine and initiation of treatment with selegiline.
-
PRECAUTIONS
General
Some patients given selegiline may experience an exacerbation of levodopa associated side effects, presumably due to the increased amounts of dopamine reaction with super sensitive, post-synaptic receptors. These effects may often be mitigated by reducing the dose of levodopa/carbidopa by approximately 10 to 30%.
The decision to prescribe selegiline should take into consideration that the MAO system of enzymes is complex and incompletely understood and there is only a limited amount of carefully documented clinical experience with selegiline. Consequently, the full spectrum of possible responses to selegiline may not have been observed in pre-marketing evaluation of the drug. It is advisable, therefore, to observe patients closely for atypical responses.
Melanoma
Epidemiological studies have shown that patients with Parkinson's disease have a higher risk (2-to approximately 6-fold higher) of developing melanoma than the general population. Whether the increased risk observed was due to Parkinson's disease or other factors, such as drugs used to treat Parkinson's disease, is unclear. For the reasons stated above, patients and providers are advised to monitor for melanomas frequently and on a regular basis when using selegiline for any indication. Ideally, periodic skin examinations should be performed by appropriately qualified individuals (e.g., dermatologists).
Information for Patients
Patients should be advised of the possible need to reduce levodopa dosage after the initiation of selegiline therapy.
Patients (or their families if the patient is incompetent) should be advised not to exceed the daily recommended dose of 10 mg. The risk of using higher daily doses of selegiline should be explained, and a brief description of the `cheese reaction' provided. Rare hypertensive reactions with selegiline at recommended doses associated with dietary influences have been reported.
Consequently, it may be useful to inform patients (or their families) about the signs and symptoms associated with MAOI induced hypertensive reactions. In particular, patients should be urged to report, immediately, any severe headache or other atypical or unusual symptoms not previously experienced.
There have been reports of patients experiencing intense urges to gamble, increased sexual urges, other intense urges and the inability to control these urges while taking one or more of the medications that increase central dopaminergic tone, that are generally used for the treatment of Parkinson’s disease, including selegiline. Although it is not proven that the medications caused these events, these urges were reported to have stopped in some cases when the dose was reduced or the medication was stopped. Prescribers should ask patients about the development of new or increased gambling urges, sexual urges or other urges while being treated with selegiline. Patients should inform their physician if they experience new or increased gambling urges, increased sexual urges or other intense urges while taking selegiline. Physicians should consider dose reduction or stopping the medication if a patient develops such urges while taking selegiline.
Laboratory Tests
No specific laboratory tests are deemed essential for the management of patients on selegiline. Periodic routine evaluation of all patients, however, is appropriate.
Drug Interactions
The occurrence of stupor, muscular rigidity, severe agitation, and elevated temperature has been reported in some patients receiving the combination of selegiline and meperidine. Symptoms usually resolve over days when the combination is discontinued. This is typical of the interaction of meperidine and MAOIs. Other serious reactions (including severe agitation, hallucinations, and death) have been reported in patients receiving this combination (see CONTRAINDICATIONS). Severe toxicity has also been reported in patients receiving the combination of tricyclic antidepressants and selegiline and selective serotonin reuptake inhibitors and selegiline. (See WARNINGS for details.) One case of hypertensive crisis has been reported in a patient taking the recommended doses of selegiline and a sympathomimetic medication (ephedrine).
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Assessment of the carcinogenic potential of selegiline in mice and rats is ongoing.
Selegiline did not induce mutations or chromosomal damage when tested in the bacterial mutation assay in Salmonella typhimurium and in an in vivo chromosomal aberration assay. While these studies provide some reassurance that selegiline is not mutagenic or clastogenic, they are not definitive because of methodological limitations. No definitive in vitro chromosomal aberration or in vitro mammalian gene mutation assays have been performed.
The effect of selegiline on fertility has not been adequately assessed.
Pregnancy
Pregnancy Category C
No teratogenic effects were observed in a study of embryo-fetal development in Sprague-Dawley rats at oral doses of 4, 12, and 36 mg/kg or 4, 12 and 35 times the human therapeutic dose on a mg/m2 basis. No teratogenic effects were observed in a study of embryo-fetal development in New Zealand White rabbits at oral doses of 5, 25, and 50 mg/kg or 10, 48, and 95 times the human therapeutic dose on a mg/m2 basis; however, in this study, the number of litters produced at the two higher doses was less than recommended for assessing teratogenic potential. In the rat study, there was a decrease in fetal body weight at the highest dose tested. In the rabbit study, increases in total resorptions and % post-implantation loss, and a decrease in the number of live fetuses per dam occurred at the highest dose tested. In a peri- and postnatal development study in Sprague-Dawley rats (oral doses of 4, 16, and 64 mg/kg or 4, 15, and 62 times the human therapeutic dose on a mg/m2 basis), an increase in the number of stillbirths and decreases in the number of pups per dam, pup survival, and pup body weight (at birth and throughout the lactation period) were observed at the two highest doses. At the highest dose tested, no pups born alive survived to Day 4 postpartum. Postnatal development at the highest dose tested in dams could not be evaluated because of the lack of surviving pups. The reproductive performance of the untreated offspring was not assessed.
There are no adequate and well-controlled studies in pregnant women. Selegiline should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
-
ADVERSE REACTIONS
Introduction
The number of patients who received selegiline in prospectively monitored pre-marketing studies is limited. While other sources of information about the use of selegiline are available (e.g., literature reports, foreign post-marketing reports, etc.) they do not provide the kind of information necessary to estimate the incidence of adverse events. Thus, overall incidence figures for adverse reactions associated with the use of selegiline cannot be provided. Many of the adverse reactions seen have also been reported as symptoms of dopamine excess.
Moreover, the importance and severity of various reactions reported often cannot be ascertained. One index of relative importance, however, is whether or not a reaction caused treatment discontinuation. In prospective pre-marketing studies, the following events led, in decreasing order of frequency, to discontinuation of treatment with selegiline: nausea, hallucinations, confusion, depression, loss of balance, insomnia, orthostatic hypotension, increased akinetic involuntary movements, agitation, arrhythmia, bradykinesia, chorea, delusions, hypertension, new or increased angina pectoris, and syncope. Events reported only once as a cause of discontinuation are ankle edema, anxiety, burning lips/mouth, constipation, drowsiness/lethargy, dystonia, excess perspiration, increased freezing, gastrointestinal bleeding, hair loss, increased tremor, nervousness, weakness, and weight loss.
Experience with selegiline obtained in parallel, placebo controlled, randomized studies provides only a limited basis for estimates of adverse reaction rates. The following reactions that occurred with greater frequency among the 49 patients assigned to selegiline as compared to the 50 patients assigned to placebo in the only parallel, placebo controlled trial performed in patients with Parkinson's disease are shown in the following Table. None of these adverse reactions led to a discontinuation of treatment.
INCIDENCE OF TREATMENT-EMERGENT ADVERSE EXPERIENCES IN THE PLACEBO-CONTROLLED CLINICAL TRIAL Adverse Event Number of Patients Reporting Events selegiline hydrochloride
N = 49Placebo
N = 50Nausea 10 3 Dizziness/Lightheaded/Fainting 7 1 Abdominal Pain 4 2 Confusion 3 0 Hallucinations 3 1 Dry mouth 3 1 Vivid Dreams 2 0 Dyskinesias 2 5 Headache 2 1 The following events were reported once in either or both groups: Ache, generalized 1 0 Anxiety/Tension 1 1 Anemia 0 1 Diarrhea 1 0 Hair Loss 0 1 Insomnia 1 1 Lethargy 1 0 Leg pain 1 0 Low back pain 1 0 Malaise 0 1 Palpitations 1 0 Urinary Retention 1 0 Weight Loss 1 0 In all prospectively monitored clinical investigations, enrolling approximately 920 patients, the following adverse events, classified by body system, were reported.
Central Nervous System
Motor/Coordination/Extrapyramidal
increased tremor, chorea, loss of balance, restlessness, blepharospasm, increased bradykinesia, facial grimace, falling down, heavy leg, muscle twitch*, myoclonic jerks*, stiff neck, tardive dyskinesia, dystonic symptoms, dyskinesia, involuntary movements, freezing, festination, increased apraxia, muscle cramps.
Mental Status/Behavioral/Psychiatric
hallucinations, dizziness, confusion, anxiety, depression, drowsiness, behavior/mood change, dreams/nightmares, tiredness, delusions, disorientation, lightheadedness, impaired memory*, increased energy*, transient high*, hollow feeling, lethargy/malaise, apathy, overstimulation, vertigo, personality change, sleep disturbance, restlessness, weakness, transient irritability.
Cardiovascular
orthostatic hypotension, hypertension, arrhythmia, palpitations, new or increased angina pectoris, hypotension, tachycardia, peripheral edema, sinus bradycardia, syncope.
Gastrointestinal
nausea/vomiting, constipation, weight loss, anorexia, poor appetite, dysphagia, diarrhea, heartburn, rectal bleeding, bruxism*, gastrointestinal bleeding (exacerbation of preexisting ulcer disease).
Genitourinary/Gynecologic/Endocrine
slow urination, transient anorgasmia*, nocturia, prostatic hypertrophy, urinary hesitancy, urinary retention, decreased penile sensation*, urinary frequency.
* indicates events reported only at doses greater than 10 mg/day.
Skin and Appendages
increased sweating, diaphoresis, facial hair, hair loss, hematoma, rash, photosensitivity.
-
OVERDOSAGE
Selegiline
No specific information is available about clinically significant overdoses with selegiline. However, experience gained during selegiline's development reveals that some individuals exposed to doses of 600 mg of d,l-selegiline suffered severe hypotension and psychomotor agitation.
Since the selective inhibition of MAO B by selegiline hydrochloride is achieved only at doses in the range recommended for the treatment of Parkinson's disease (e.g., 10 mg/day), overdoses are likely to cause significant inhibition of both MAO A and MAO B. Consequently, the signs and symptoms of overdose may resemble those observed with marketed non-selective MAO inhibitors [e.g., tranylcypromine, isocarboxazide, and phenelzine].
Overdose with Non-Selective MAO Inhibition
NOTE: This section is provided for reference; it does not describe events that have actually been observed with selegiline in overdose.
Characteristically, signs and symptoms of non‑selective MAOI overdose may not appear immediately. Delays of up to 12 hours between ingestion of drug and the appearance of signs may occur. Importantly, the peak intensity of the syndrome may not be reached for upwards of a day following the overdose. Death has been reported following overdosage. Therefore, immediate hospitalization, with continuous patient observation and monitoring for a period of at least two days following the ingestion of such drugs in overdose, is strongly recommended.
The clinical picture of MAOI overdose varies considerably; its severity may be a function of the amount of drug consumed. The central nervous and cardiovascular systems are prominently involved.
Signs and symptoms of overdosage may include, alone or in combination, any of the following: drowsiness, dizziness, faintness, irritability, hyperactivity, agitation, severe headache, hallucinations, trismus, opisthotonus, convulsions, and coma; rapid and irregular pulse, hypertension, hypotension and vascular collapse; precordial pain, respiratory depression and failure, hyperpyrexia, diaphoresis, and cool, clammy skin.
Treatment Suggestions For Overdose
NOTE: Because there is no recorded experience with selegiline overdose, the following suggestions are offered based upon the assumption that selegiline overdose may be modeled by non-selective MAOI poisoning. In any case, up-to-date information about the treatment of overdose can often be obtained from a certified Regional Poison Control Center. Telephone numbers of certified Poison Control Centers are listed in the Physicians' Desk Reference (PDR).
Treatment of overdose with non-selective MAOIs is symptomatic and supportive. Induction of emesis or gastric lavage with instillation of charcoal slurry may be helpful in early poisoning, provided the airway has been protected against aspiration. Signs and symptoms of central nervous system stimulation, including convulsions, should be treated with diazepam, given slowly intravenously. Phenothiazine derivatives and central nervous system stimulants should be avoided. Hypotension and vascular collapse should be treated with intravenous fluids and, if necessary, blood pressure titration with an intravenous infusion of a dilute pressor agent. It should be noted that adrenergic agents may produce a markedly increased pressor response.
Respiration should be supported by appropriate measures, including management of the airway, use of supplemental oxygen, and mechanical ventilatory assistance, as required.
Body temperature should be monitored closely. Intensive management of hyperpyrexia may be required. Maintenance of fluid and electrolyte balance is essential.
-
DOSAGE AND ADMINISTRATION
Selegiline hydrochloride capsules are intended for administration to Parkinsonian patients receiving levodopa/carbidopa therapy who demonstrate a deteriorating response to this treatment. The recommended regimen for the administration of selegiline hydrochloride is 10 mg per day administered as divided doses of 5 mg each taken at breakfast and lunch. There is no evidence that additional benefit will be obtained from the administration of higher doses. Moreover, higher doses should ordinarily be avoided because of the increased risk of side effects.
After two to three days of selegiline treatment, an attempt may be made to reduce the dose of levodopa/carbidopa. A reduction of 10 to 30% was achieved with the typical participant in the domestic placebo controlled trials who was assigned to selegiline treatment. Further reductions of levodopa/carbidopa may be possible during continued selegiline therapy.
-
HOW SUPPLIED
Selegiline Hydrochloride Capsules, USP 5 mg are available for oral administration as hard gelatin capsules with a white opaque body and an aqua blue opaque cap. “APO 055” is imprinted on each capsule in black ink. They are supplied as bottles of 60 (NDC 60505-0055-1), bottles of 500 (NDC 60505-0055-3) and bottles of 1000 (NDC 60505-0055-2).
Storage
Store at 20º to 25ºC (68º to 77Fº) [see USP Controlled Room Temperature].
APOTEX INC.
SELEGILINE HYDROCHLORIDE CAPSULES, USP 5 MG
Manufactured by: Manufactured for: Apotex Inc. Apotex Corp. Toronto, Ontario Weston, Florida Canada 33326 M9L 1T9 Revised: June 2014
Rev. 5
-
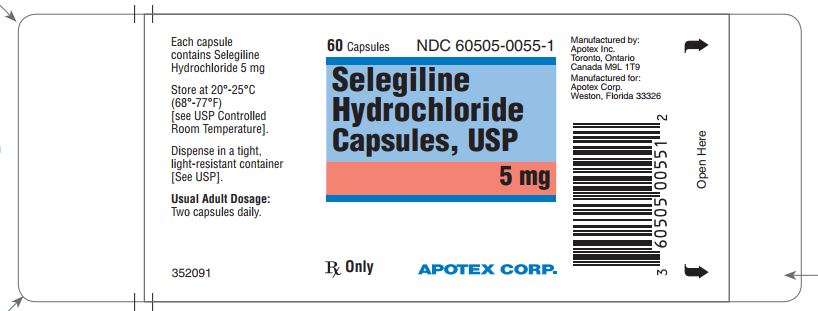
PRINCIPAL DISPLAY PANEL SECTION
Representative sample of the labeling (see the HOW SUPPLIED section for complete listing):
PRINCIPAL DISPLAY PANEL - 5 mg BOTTLE LABEL
APOTEX CORP. NDC 60505-0055-1
SELEGILINE HYDROCHLORIDE CAPSULES,
5 mg
Rx
60 Capsules
-
INGREDIENTS AND APPEARANCE
SELEGILINE HYDROCHLORIDE
selegiline hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:60505-0055 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Selegiline Hydrochloride (UNII: 6W731X367Q) (Selegiline - UNII:2K1V7GP655) Selegiline Hydrochloride 5 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) AMMONIA (UNII: 5138Q19F1X) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ALCOHOL (UNII: 3K9958V90M) ISOPROPYL ALCOHOL (UNII: ND2M416302) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SHELLAC (UNII: 46N107B71O) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) Product Characteristics Color WHITE, BLUE Score no score Shape CAPSULE Size 16mm Flavor Imprint Code APO;055 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60505-0055-1 60 in 1 BOTTLE; Type 0: Not a Combination Product 07/15/1997 2 NDC:60505-0055-3 500 in 1 BOTTLE; Type 0: Not a Combination Product 07/15/1997 3 NDC:60505-0055-2 1000 in 1 BOTTLE; Type 0: Not a Combination Product 07/15/1997 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075321 07/15/1997 Labeler - Apotex Corp. (845263701) Registrant - Apotex Inc. (205576023) Establishment Name Address ID/FEI Business Operations Apotex Inc. 209429182 manufacture(60505-0055) , analysis(60505-0055)