Label: SAMSALI WART CORN REMOVER PADS- wart corn remover pads patch
- NDC Code(s): 83818-004-01
- Packager: Shenzhen Xinxin Yunhai Technology Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- When Using
- Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
- Directions
- Other information
- Inactive ingredients
- Questions
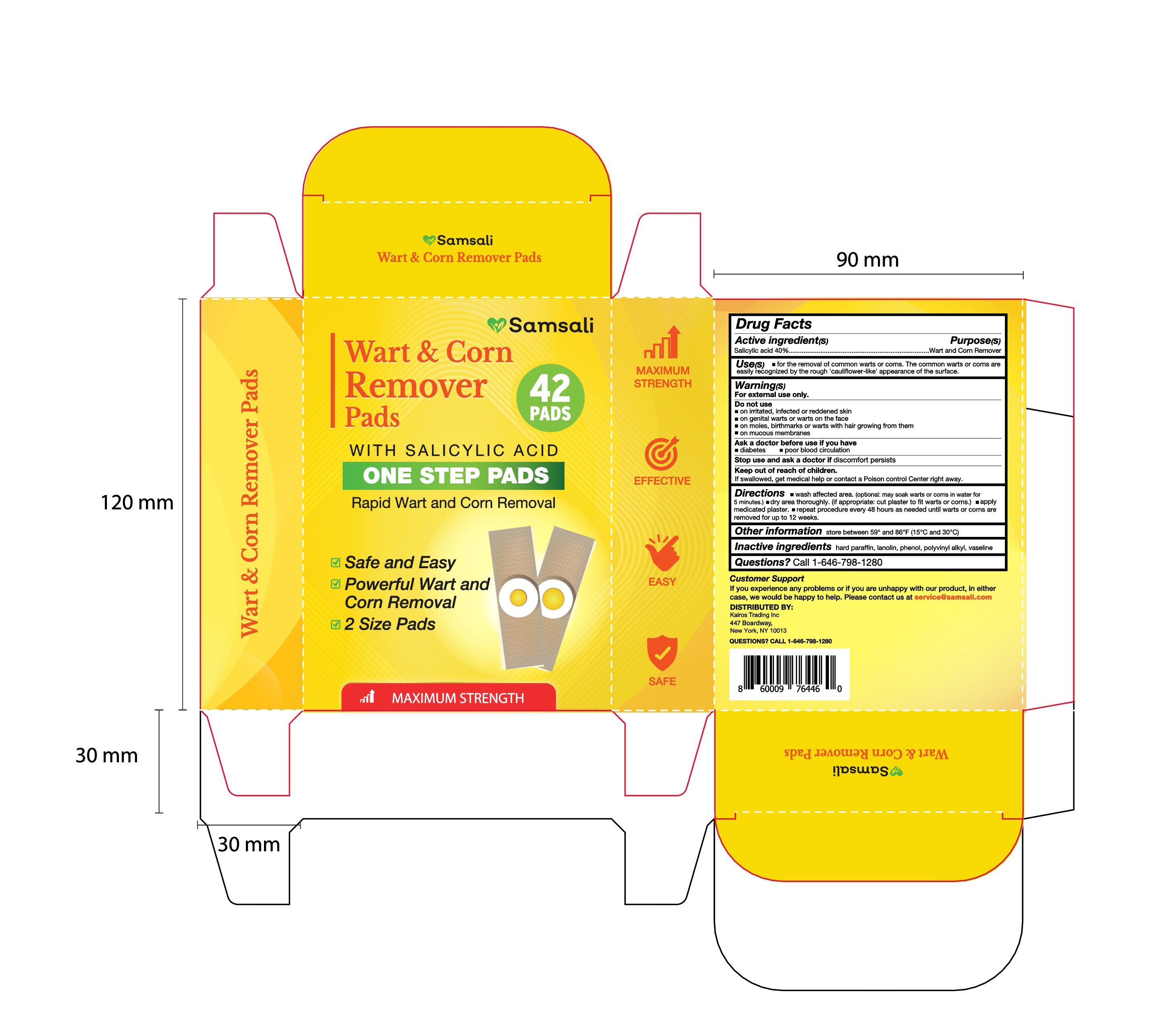
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SAMSALI WART CORN REMOVER PADS
wart corn remover pads patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83818-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 40 g in 100 Inactive Ingredients Ingredient Name Strength CLEMATIS VIORNA WHOLE (UNII: DNP609X607) PARAFFIN (UNII: I9O0E3H2ZE) PHENOL (UNII: 339NCG44TV) LANOLIN (UNII: 7EV65EAW6H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83818-004-01 42 in 1 BOX; Type 0: Not a Combination Product 05/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M030 05/14/2024 Labeler - Shenzhen Xinxin Yunhai Technology Co., Ltd. (699816806) Establishment Name Address ID/FEI Business Operations Shenzhen Xinxin Yunhai Technology Co., Ltd. 699816806 manufacture(83818-004)