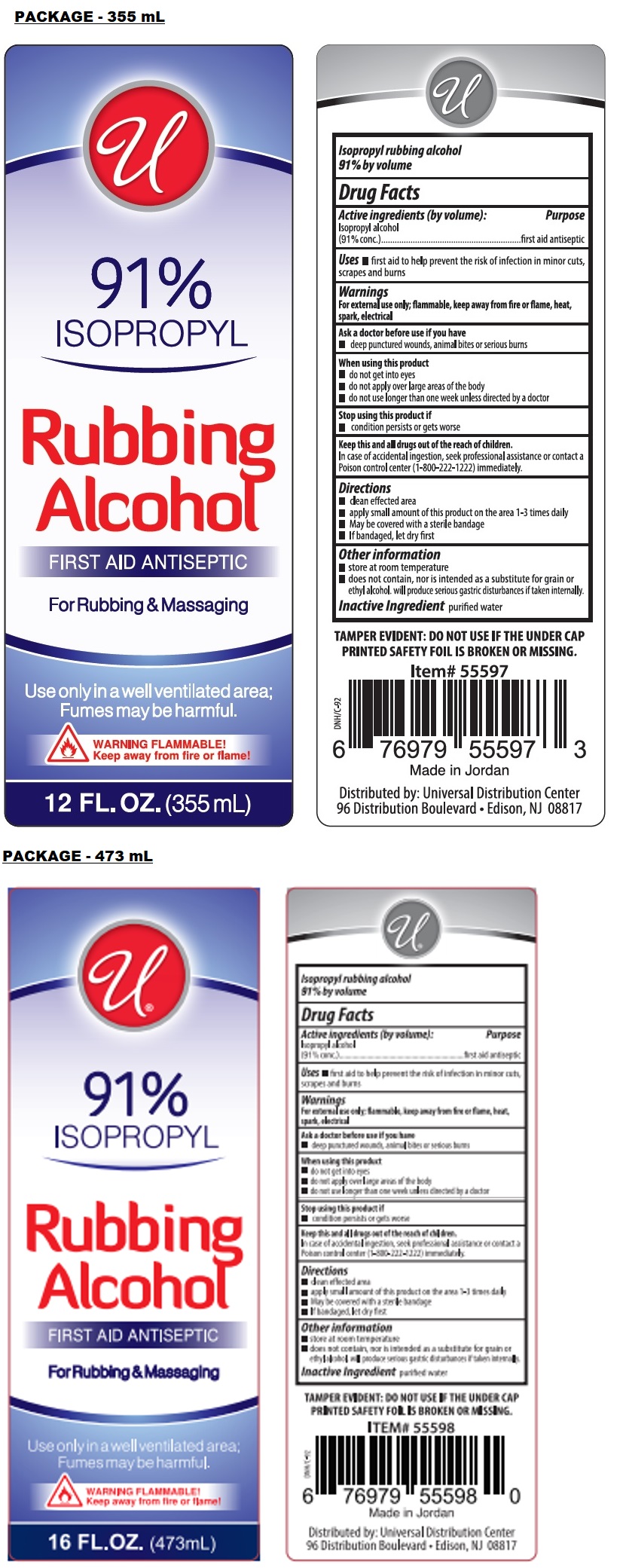
Label: UNIVERSAL RUBBING ALCOHOL- isopropyl alcohol liquid
- NDC Code(s): 52000-406-35, 52000-406-36
- Packager: Universal Distribution Center LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients (by volume):
- Purpose
- Uses
-
Warnings
For external use only; flammable, keep away from fire or flame, heat, spark, electrical
Ask a doctor before use if you have
• deep punctured wounds, animal bites or serious burnsWhen using this product
• do not get into eyes
• do not apply over large areas of the body
• do not use longer than one week unless directed by a doctorStop using this product if
• condition persists or gets worse - Directions
- Other information
- Inactive Ingredient
- SPL UNCLASSIFIED SECTION
- Packaging
-
INGREDIENTS AND APPEARANCE
UNIVERSAL RUBBING ALCOHOL
isopropyl alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52000-406 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 91 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52000-406-35 355 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/01/2023 2 NDC:52000-406-36 473 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 06/01/2023 Labeler - Universal Distribution Center LLC (019180459)