Label: PRAZOSIN HYDROCHLORIDE capsule
- NDC Code(s): 70518-2705-0, 70518-2705-1, 70518-2705-2, 70518-2705-3
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 70954-021
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 26, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
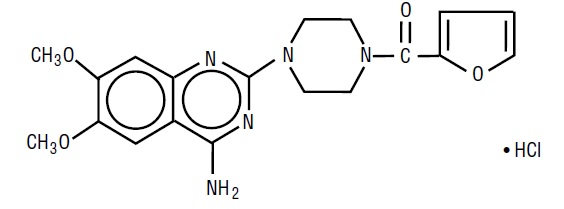
DESCRIPTIONPrazosin hydrochloride, USP a quinazoline derivative, is the first of a new chemical class of antihypertensives. It is the hydrochloride salt of ...
-
CLINICAL PHARMACOLOGYThe exact mechanism of the hypotensive action of prazosin is unknown. Prazosin causes a decrease in total peripheral resistance and was originally thought to have a direct relaxant action on ...
-
INDICATIONS & USAGEPrazosin Hydrochloride Capsules, USP is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events ...
-
CONTRAINDICATIONSPrazosin Hydrochloride Capsules are contraindicated in patients with known sensitivity to quinazolines, prazosin, or any of the inert ingredients.
-
WARNINGSAs with all alpha-blockers, prazosin hydrochloride may cause syncope with sudden loss of consciousness. In most cases, this is believed to be due to an excessive postural hypotensive effect ...
-
PRECAUTIONSGENERAL PRECAUTIONS - Intraoperative Floppy Iris Syndrome (IFIS) has been observed during cataract surgery in some patients treated with alpha-1 blockers. This variant of small pupil syndrome is ...
-
ADVERSE REACTIONSClinical trials were conducted on more than 900 patients. During these trials and subsequent marketing experience, the most frequent reactions associated with prazosin hydrochloride therapy are ...
-
OVERDOSAGEAccidental ingestion of at least 50 mg of prazosin hydrochloride in a two year old child resulted in profound drowsiness and depressed reflexes. No decrease in blood pressure was noted. Recovery ...
-
DOSAGE & ADMINISTRATIONThe dose of prazosin hydrochloride capsules should be adjusted according to the patient’s individual blood pressure response. The following is a guide to its administration: Initial Dose - 1 ...
-
HOW SUPPLIEDPrazosin Hydrochloride Capsules, USP 5 mg for oral administration containing prazosin hydrochloride, USP equivalent to 5 mg of prazosin, are supplied as follows: Hard gelatin capsule shell with ...
-
REFERENCES1) Lubbe, WF, and Hodge, JV: New Zealand Med J, 94 (691) 169-172,1981. 2) Davey, DA, and Dommisse, J: SA. Med J, Oct.4, 1980 (551-556).
-
PRINCIPAL DISPLAY PANELDRUG: Prazosin Hydrochloride - GENERIC: Prazosin Hydrochloride - DOSAGE: CAPSULE - ADMINSTRATION: ORAL - NDC: 70518-2705-0 - NDC: 70518-2705-1 - NDC: 70518-2705-2 - NDC: 70518-2705-3 - COLOR: blue - SHAPE ...
-
INGREDIENTS AND APPEARANCEProduct Information