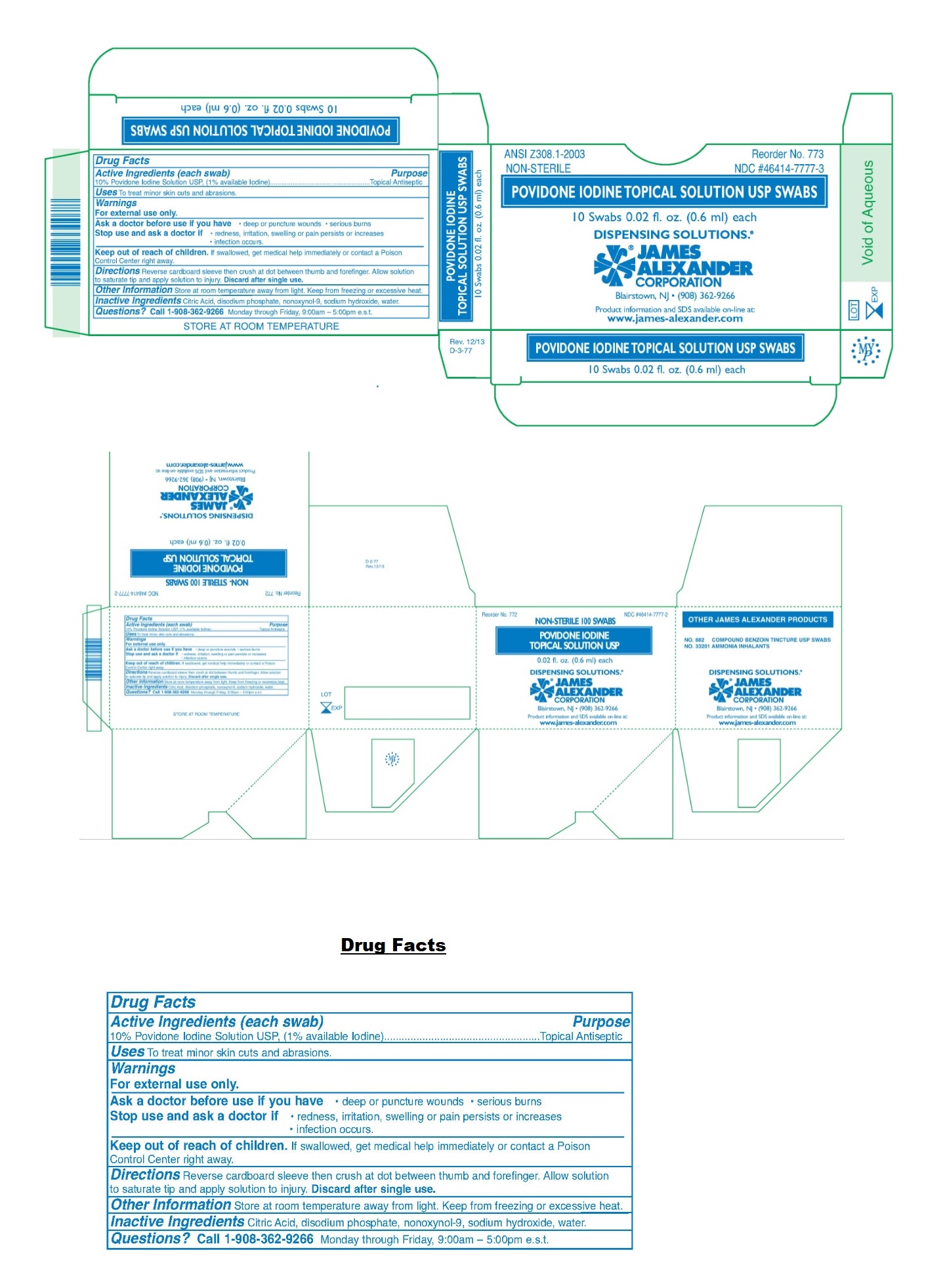
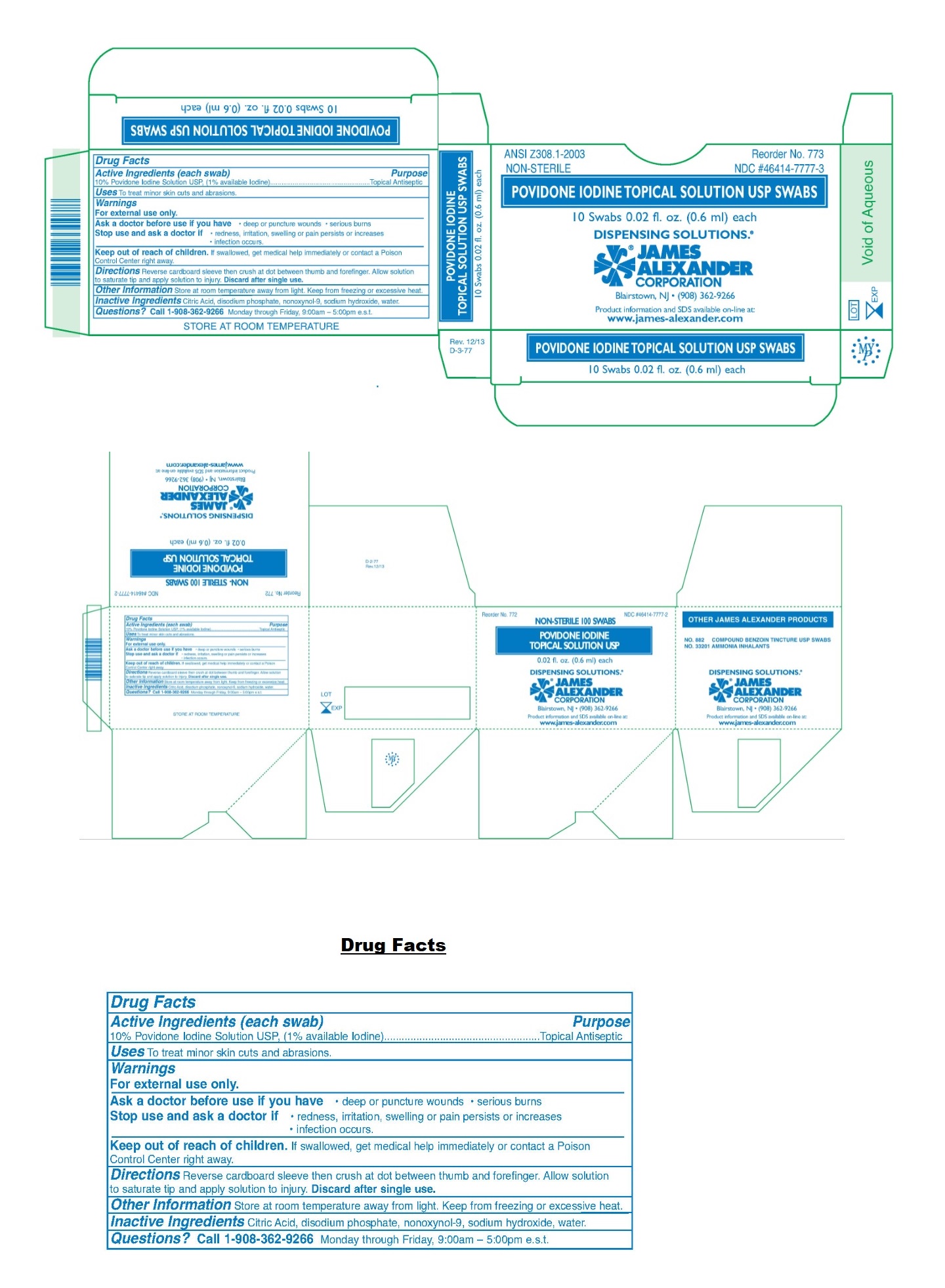
Label: POVIDONE-IODINE solution
- NDC Code(s): 46414-7777-2, 46414-7777-3
- Packager: James Alexander Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients (each swab)
- Purpose
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other Information
- Inactive Ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- Packaging
-
INGREDIENTS AND APPEARANCE
POVIDONE-IODINE
povidone-iodine solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:46414-7777 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) NONOXYNOL-9 (UNII: 48Q180SH9T) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46414-7777-3 10 in 1 CONTAINER 02/14/1976 1 0.6 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 2 NDC:46414-7777-2 100 in 1 CONTAINER 02/14/1976 2 0.6 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 02/14/1976 Labeler - James Alexander Corporation (040756421) Registrant - James Alexander Corporation (040756421) Establishment Name Address ID/FEI Business Operations James Alexander Corporation 040756421 manufacture(46414-7777)