Label: CLEARASIL STUBBORN ACNE CONTROL 5IN1 DAILY PADS- salicylic acid cloth
- NDC Code(s): 63824-439-90
- Packager: RB Health (US) LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
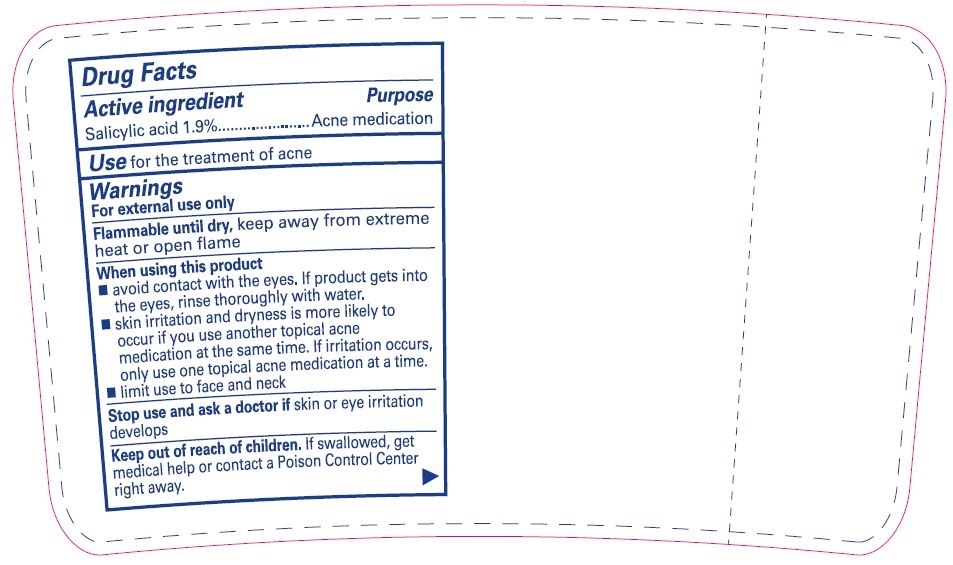
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only
When using this product
- avoid contact with the eyes. If product gets into the eyes rinse thoroughly with water.
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- limit use to face and neck
-
Directions
- clean the skin thoroughly before applying this product
- wipe a pad over face and neck to cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 90 Pad Jar Label
-
INGREDIENTS AND APPEARANCE
CLEARASIL STUBBORN ACNE CONTROL 5IN1 DAILY PADS
salicylic acid clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63824-439 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.03 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) ISOCETETH-20 (UNII: O020065R7Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) SODIUM HYDROXIDE (UNII: 55X04QC32I) TROLAMINE (UNII: 9O3K93S3TK) ALLANTOIN (UNII: 344S277G0Z) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63824-439-90 90 in 1 JAR; Type 4: Device Coated/Impregnated/Otherwise Combined with Drug 12/05/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/05/2017 Labeler - RB Health (US) LLC (081049410)