Label: ALBUTEROL SULFATE inhalant
- NDC Code(s): 17270-0740-0
- Packager: Armstrong Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ALBUTEROL SULFATE INHALATION AEROSOL safely and effectively. See full prescribing information for ALBUTEROL SULFATE INHALATION ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Bronchospasm - Albuterol Sulfate Inhalation Aerosol is indicated for the treatment or prevention of bronchospasm in patients 4 years of age and older with reversible obstructive airway ...
-
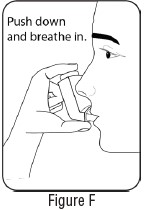
2 DOSAGE AND ADMINISTRATION2.1 Bronchospasm - For treatment of acute episodes of bronchospasm or prevention of symptoms associated with bronchospasm, the usual dosage for adults and children 4 years and older is two ...
-
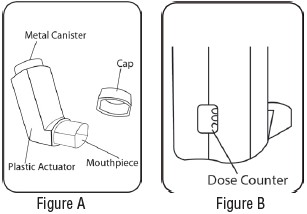
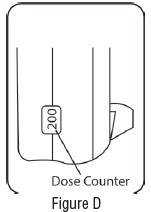
3 DOSAGE FORMS & STRENGTHSAlbuterol Sulfate is an inhalation aerosol. Albuterol Sulfate Inhalation Aerosol is supplied as an 8.5 g/200 actuations pressurized aluminum canister with a green plastic actuator with a dose ...
-
4 CONTRAINDICATIONSAlbuterol Sulfate Inhalation Aerosol is contraindicated in patients with a history of hypersensitivity to albuterol and any other Albuterol Sulfate Inhalation Aerosol components. Rare cases of ...
-
5 WARNINGS & PRECAUTIONS5.1 Paradoxical Bronchospasm - Albuterol Sulfate Inhalation Aerosol can produce paradoxical bronchospasm that may be life threatening. If paradoxical bronchospasm occurs, Albuterol Sulfate ...
-
6 ADVERSE REACTIONSUse of Albuterol Sulfate may be associated with the following: Paradoxical bronchospasm [see Warnings and Precations (5.1)] Cardiovascular Effects [see Warnings and Precations (5.4)] Immediate ...
-
7 DRUG INTERACTIONSOther short-acting sympathomimetic aerosol bronchodilators should not be used concomitantly with Albuterol Sulfate Inhalation Aerosol. If additional adrenergic drugs are to be administered by any ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to asthma medications during pregnancy. For more information ...
-
10 OVERDOSAGEThe expected symptoms with overdosage are those of excessive beta-adrenergic stimulation and/or occurrence or exaggeration of any of the symptoms listed under ADVERSE REACTIONS, e.g., seizures ...
-
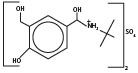
11 DESCRIPTIONThe active ingredient of Albuterol Sulfate Inhalation Aerosol is albuterol sulfate, a racemic salt, of albuterol. Albuterol sulfate has the chemical name α1-[(tert-butylamino ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Albuterol sulfate is a beta2-adrenergic agonist. The pharmacologic effects of albuterol sulfate are attributable to activation of beta2-adrenergic receptors on airway ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a 2-year study in Sprague-Dawley rats, albuterol sulfate caused a dose-related increase in the incidence of benign leiomyomas of the ...
-
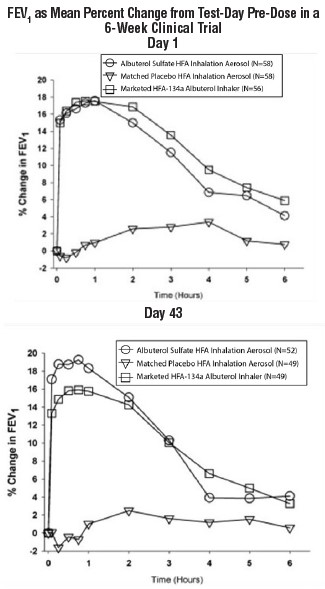
14 CLINICAL STUDIES14.1 Bronchospasm Associated with Asthma - Adult and Adolescent Patients 12 Years of Age and Older: In a 6-week, randomized, double-blind, placebo-controlled trial, Albuterol Sulfate Inhalation ...
-
16 HOW SUPPLIED/STORAGE & HANDLINGAlbuterol Sulfate Inhalation Aerosol (albuterol sulfate) is supplied as a pressurized aluminum canister with a green plastic actuator with a dose counter and yellow dust cap each in boxes of one ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-Approved Patient Labeling (17.9) Patients should be given the following information: 17.1 Frequency of Use - The action of Albuterol Sulfate Inhalation Aerosol should last for 4 to 6 ...
-
PATIENT PACKAGE INSERTAttention Pharmacist: Detach Patient’s Instructions for use from package insert and dispense with the product. Patient Information - Albuterol Sulfate - Inhalation Aerosol - Read this ...
-
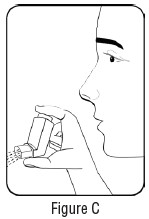
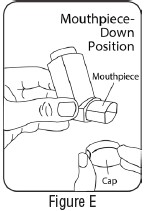
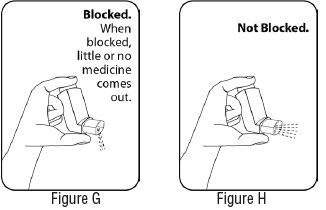
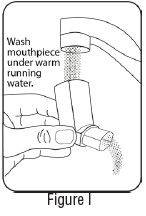
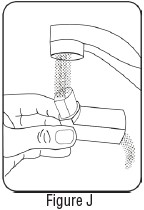
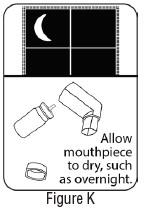
INSTRUCTIONS FOR USEInstructions for Use - Albuterol Sulfate - Inhalation Aerosol - Read this Instructions for Use before you start using Albuterol Sulfate Inhalation Aerosol and each time you get a refill. There ...
-
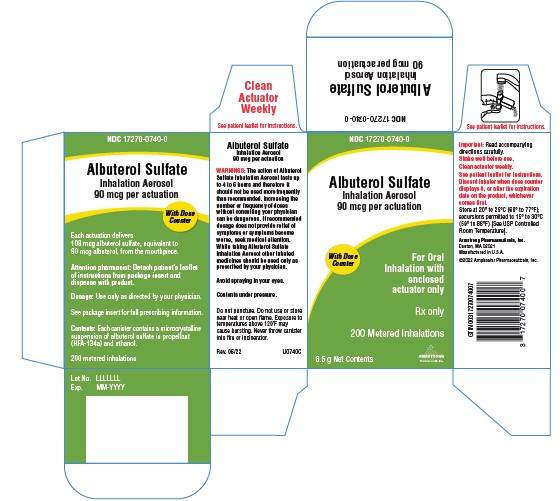
PRINCIPAL DISPLAY PANELPrincipal Display Panel Text: NDC 17270-0740-0 - Albuterol Sulfate - Inhalation Aerosol - 90 mcg per actuation - With Dose - Counter - For Oral - Inhalation with - enclosed - actuator only - Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information