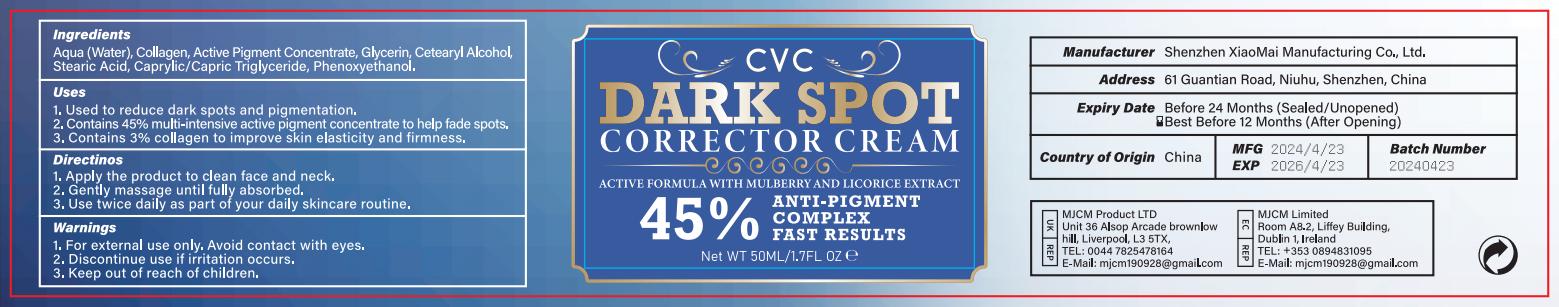
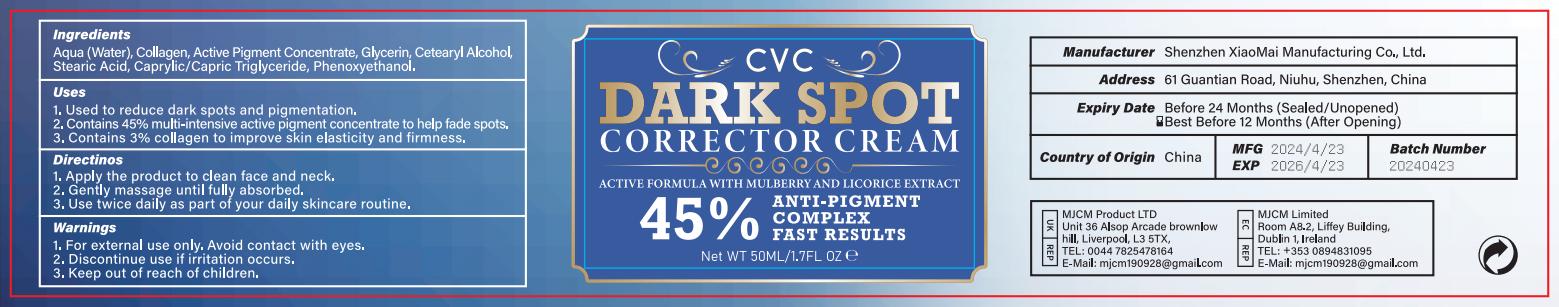
Label: DARK SPOT CORRECTOR CREAM cream
- NDC Code(s): 83872-122-01
- Packager: Shenzhen XiaoMai Manufacturing Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- Uses
- WARNINGS
- Do not use
- When using this product
- Stop use and ask a doctor
- Keep out of reach of children.
- Directions for use
- INACTIVE INGREDIENT
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DARK SPOT CORRECTOR CREAM
dark spot corrector cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83872-122 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 50 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 700 mg in 1 g STEARIC ACID (UNII: 4ELV7Z65AP) 30 mg in 1 g PHENOXYETHANOL (UNII: HIE492ZZ3T) 20 mg in 1 g Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83872-122-01 50 g in 1 BOTTLE; Type 0: Not a Combination Product 05/02/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 05/02/2024 Labeler - Shenzhen XiaoMai Manufacturing Co., Ltd. (712999147) Establishment Name Address ID/FEI Business Operations Shenzhen XiaoMai Manufacturing Co., Ltd. 712999147 manufacture(83872-122)