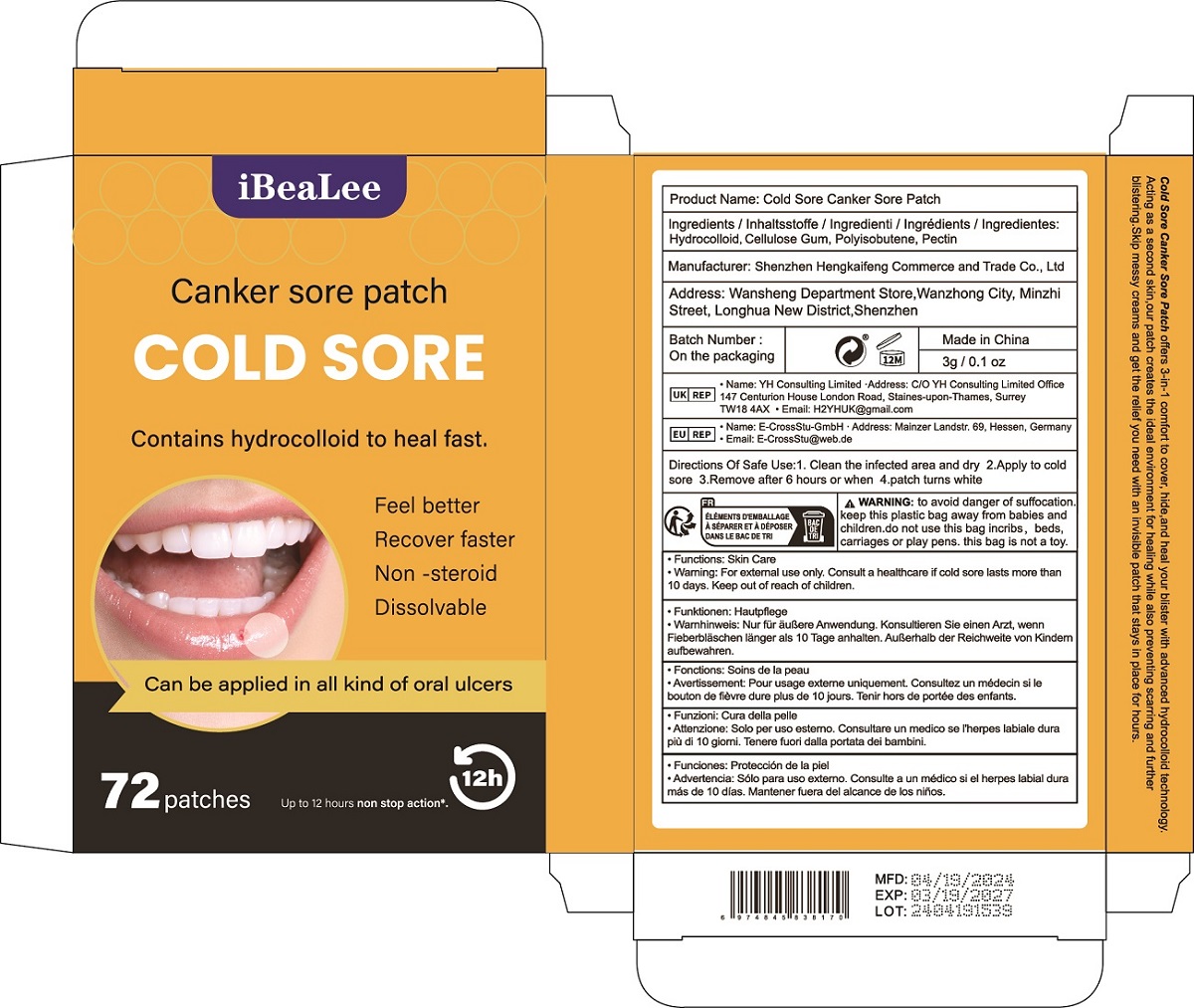
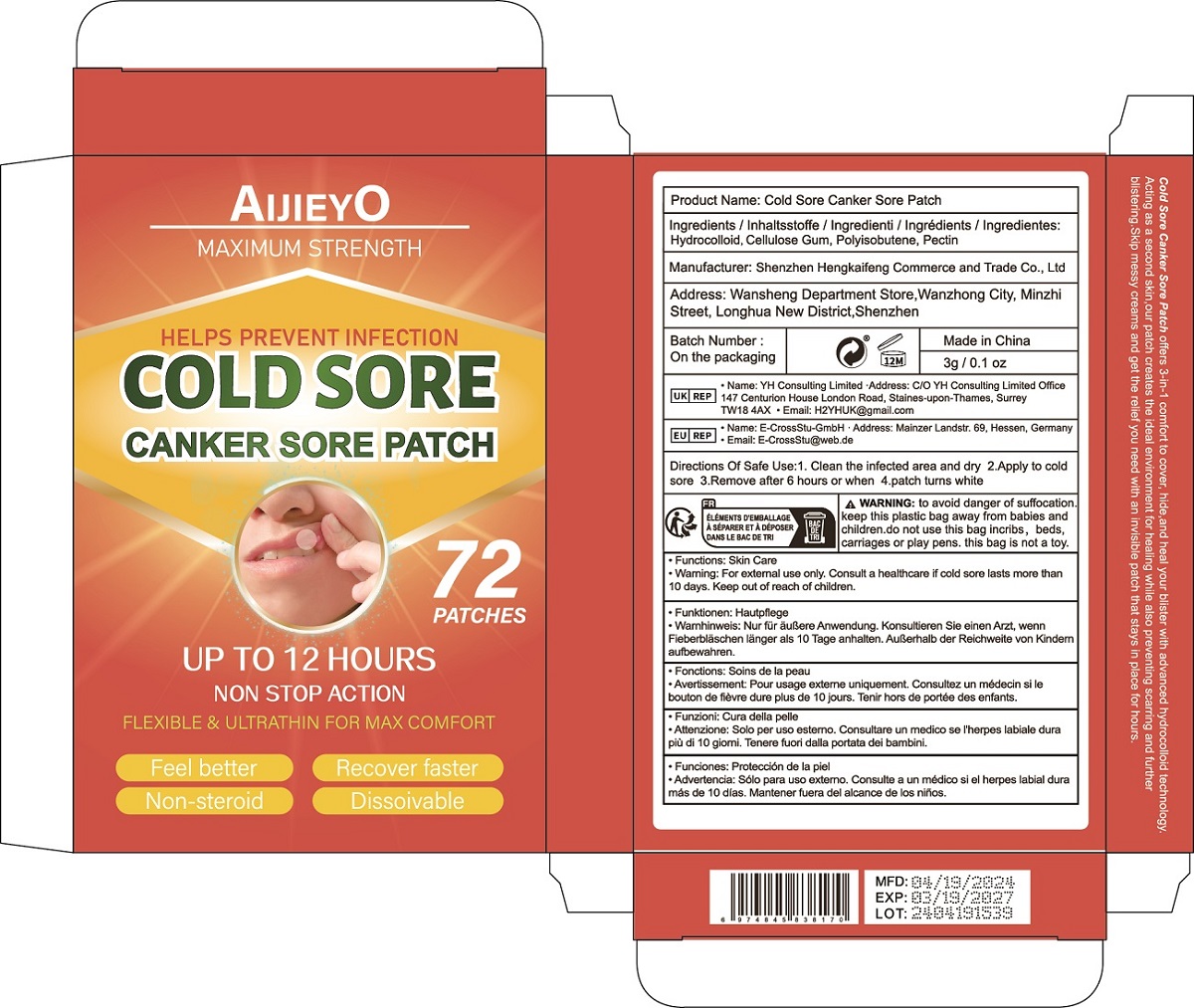
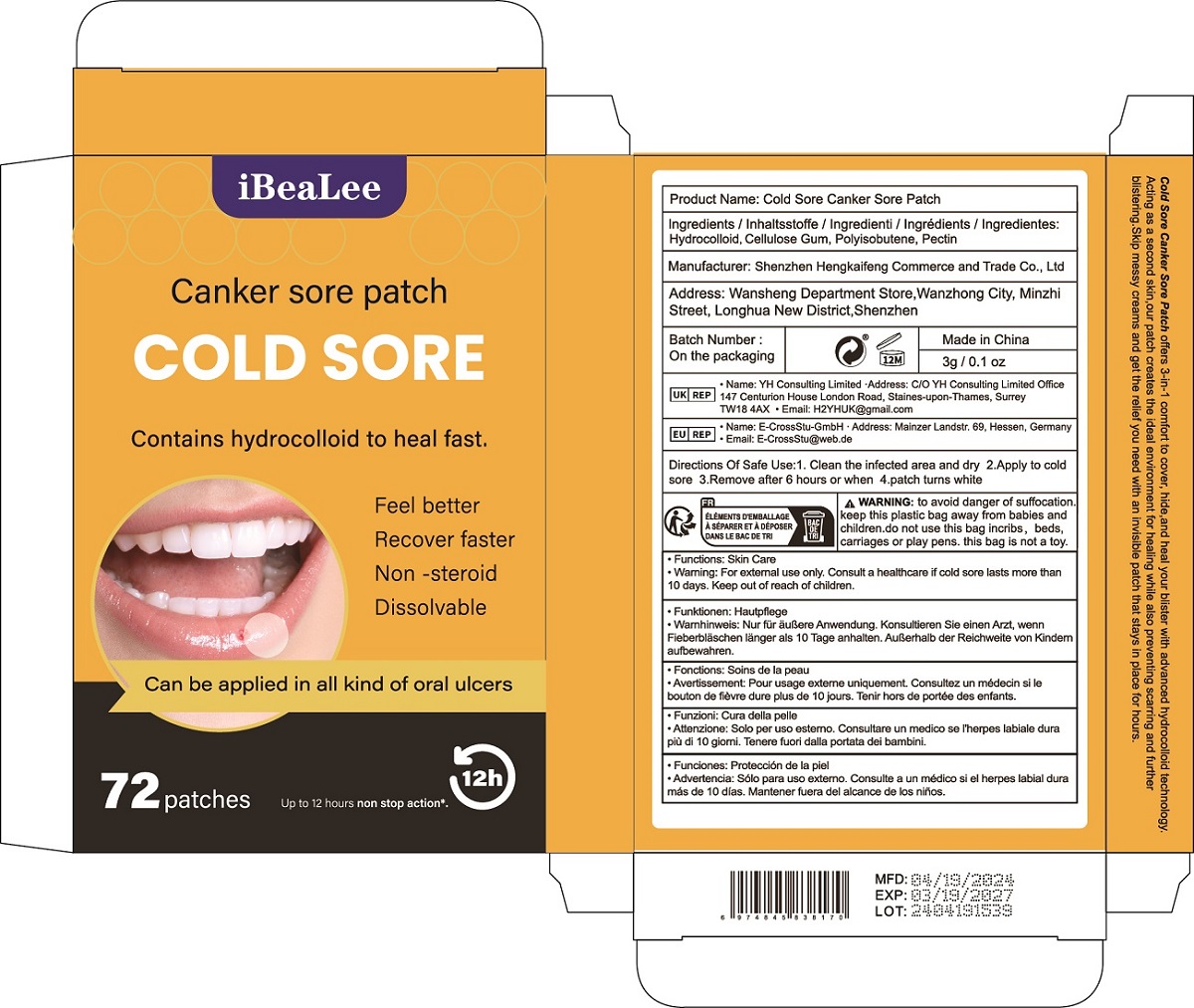
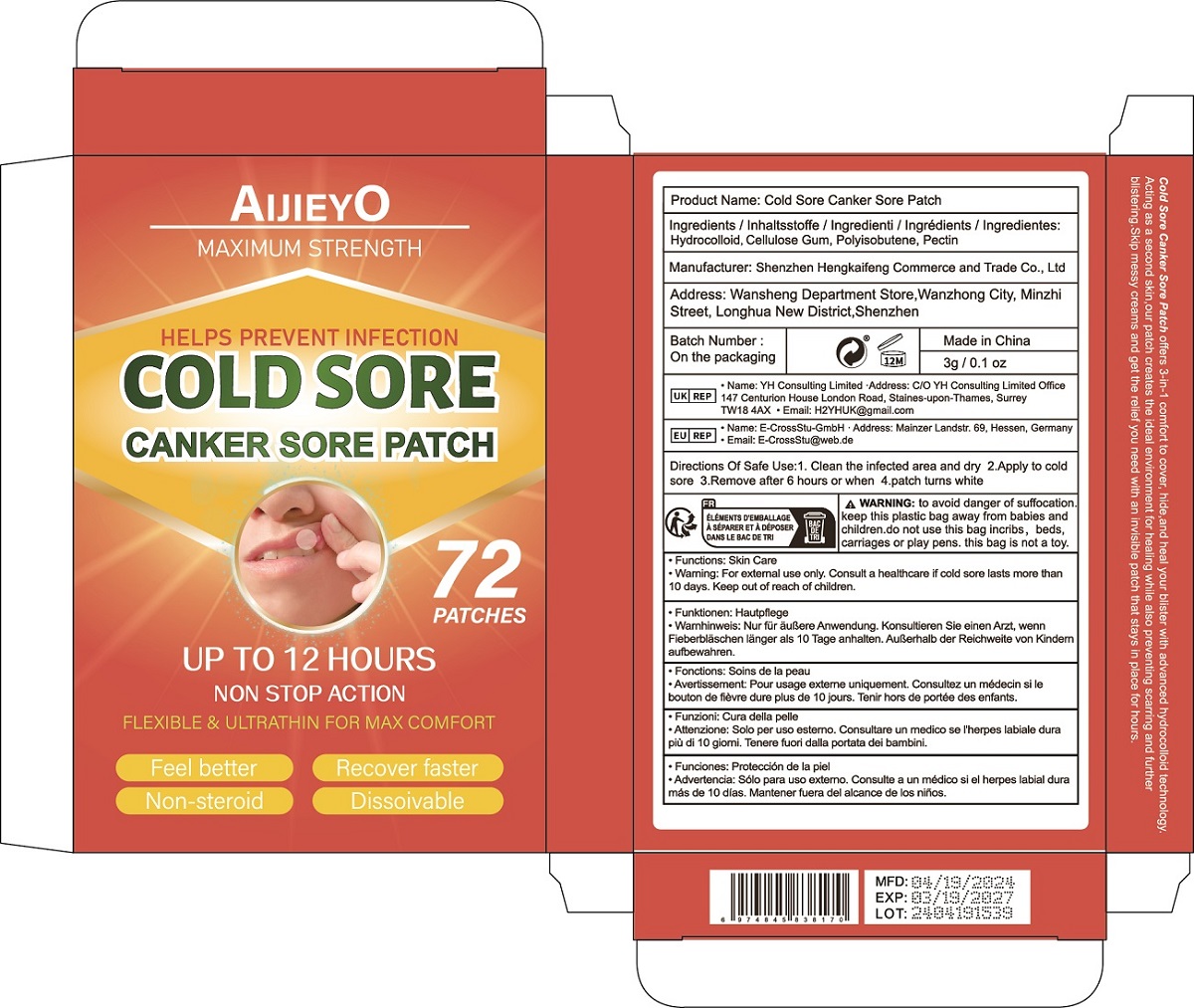
Label: COLD SORE CANKER SORE PATCH patch
- NDC Code(s): 84117-008-01
- Packager: Shenzhen Hengkaifeng Commerce and Trade Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Purpose
-
Use
Cold Sore Canker Sore Patch offers 3-in-1 comfort to cover, hide,and heal your blister with advanced hydrocolloid technology.
Acting as a second skin,our patch creates the ideal environment for healing while also preventing scarring and further
blistering.Skip messy creams and get the relief you need with an invisible patch that stays in place for hours. - Warnings
- Keep Out Of Reach Of Children
- Directions Of Safe Use:
- Inactive ingredients
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COLD SORE CANKER SORE PATCH
cold sore canker sore patch patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84117-008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PECTIN (UNII: 89NA02M4RX) (PECTIN - UNII:89NA02M4RX) PECTIN 5 g in 100 g Inactive Ingredients Ingredient Name Strength HYDROGENATED POLYISOBUTENE (450 MW) (UNII: 7YR4ZFS62E) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84117-008-01 3 g in 1 BOX; Type 0: Not a Combination Product 05/02/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 05/02/2024 Labeler - Shenzhen Hengkaifeng Commerce and Trade Co., Ltd (444722774) Establishment Name Address ID/FEI Business Operations Shenzhen Hengkaifeng Commerce and Trade Co., Ltd 444722774 label(84117-008) , manufacture(84117-008)