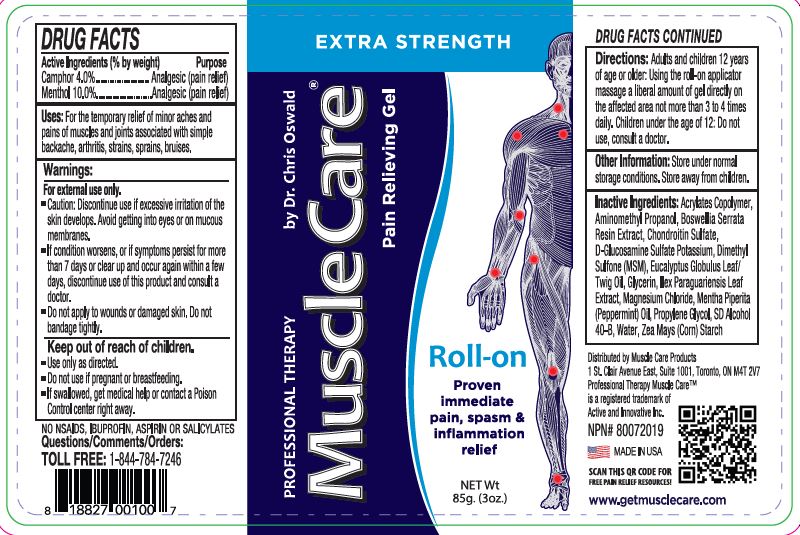
Label: PROFESSIONAL THERAPY MUSCLECARE PRO ROLL-ON- camphor, menthol gel
- NDC Code(s): 70039-301-03
- Packager: ACTIVE AND INNOVATIVE, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredietns (% by weight) Purpose
- Purpose
- Uses:
-
Warnings:
For external use only.
- Caution: Discontinue use if excessive irritation of the skin develops. Avoid getting into eyes or on mucous membranes.
- If condition worsens, or if symptoms persist for more than 7 days, discontinue use of this product and consult a doctor.
- Do not apply to wounds or damaged skin. Do not bandage tightly.
- Keep out of reach of children.
- Directions
- Other Information
-
Inactive Ingredients
Acrylates Copolymer, Aminomethyl Propanol, Boswellia Serrata Resin Extract, Chondroitin Sulfate, D-Glucosamine Sulfate
Potassium, Dimethyl Sulfone (MSM), Eucalyptus Globulus Leaf/Twig Oil, Glycerin, Ilex Paraguariensis Leaf Extract, Magnesium
Chloride, Mentha Piperita (Peppermint) Oil, Propylene Glycol, SD Alcohol 40-B, Water, Zea Mays (Corn) Starch - Professionl Therapy MuscleCare Maximum Strength Pain Relieving Gel Roll-on
-
INGREDIENTS AND APPEARANCE
PROFESSIONAL THERAPY MUSCLECARE PRO ROLL-ON
camphor, menthol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70039-301 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 4 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 10 g in 100 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) STARCH, CORN (UNII: O8232NY3SJ) EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER (60000 MPA.S) (UNII: 8Z5ZAL5H3V) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) GLYCERIN (UNII: PDC6A3C0OX) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) CHONDROITIN SULFATE (BOVINE) (UNII: 6IC1M3OG5Z) GLUCOSAMINE SULFATE POTASSIUM CHLORIDE (UNII: 15VQ11I66N) WATER (UNII: 059QF0KO0R) ILEX PARAGUARIENSIS LEAF (UNII: 1Q953B4O4F) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) MENTHA PIPERITA (UNII: 79M2M2UDA9) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70039-301-03 85 g in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 04/30/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 04/30/2024 Labeler - ACTIVE AND INNOVATIVE, LLC (206978079) Registrant - MCKENNA LABS, INC. (090631412) Establishment Name Address ID/FEI Business Operations MCKENNA LABS, INC. 090631412 manufacture(70039-301)