Label: VERQUVO- vericiguat tablet, film coated
-
NDC Code(s):
0006-5028-01,
0006-5028-02,
0006-5028-03,
0006-5028-04, view more0006-5028-05, 0006-5028-06, 0006-5029-01, 0006-5029-02, 0006-5029-03, 0006-5029-04, 0006-5029-05, 0006-5029-06, 0006-5030-01, 0006-5030-02, 0006-5030-03, 0006-5030-04
- Packager: Merck Sharp & Dohme LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VERQUVO safely and effectively. See full prescribing information for VERQUVO.
VERQUVO ® (vericiguat) tablets, for oral use
Initial U.S. Approval: 2021WARNING: EMBRYO-FETAL TOXICITY
See full prescribing information for complete boxed warning.
- Do not administer VERQUVO to a pregnant female because it may cause fetal harm. (4, 5.1, 8.1)
- Females of reproductive potential: Exclude pregnancy before the start of treatment. To prevent pregnancy, females of reproductive potential must use effective forms of contraception during treatment and for one month after stopping treatment. (2.2, 5.1, 8.3)
INDICATIONS AND USAGE
VERQUVO is a soluble guanylate cyclase (sGC) stimulator, indicated to reduce the risk of cardiovascular death and heart failure (HF) hospitalization following a hospitalization for heart failure or need for outpatient IV diuretics, in adults with symptomatic chronic HF and ejection fraction less than 45%. (1)
DOSAGE AND ADMINISTRATION
- The recommended starting dose of VERQUVO is 2.5 mg orally once daily with food. (2.1)
- Double the dose of VERQUVO approximately every 2 weeks to reach the target maintenance dose of 10 mg once daily, as tolerated by the patient. (2.1)
- Tablets may be crushed and mixed with water for patients who have difficulty swallowing. (2.1)
DOSAGE FORMS AND STRENGTHS
Tablets: 2.5 mg, 5 mg and 10 mg (3)
CONTRAINDICATIONS
ADVERSE REACTIONS
Most common adverse reactions reported in ≥5% are hypotension and anemia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- PDE-5 Inhibitors: Concomitant use is not recommended. (7.2)
USE IN SPECIFIC POPULATIONS
- Lactation: Breastfeeding is not recommended (8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 7/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: EMBRYO-FETAL TOXICITY
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Pregnancy Testing in Females of Reproductive Potential
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Other Soluble Guanylate Cyclase Stimulators
7.2 PDE-5 Inhibitors
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: EMBRYO-FETAL TOXICITY
Females of reproductive potential: Exclude pregnancy before the start of treatment. To prevent pregnancy, females of reproductive potential must use effective forms of contraception during treatment and for one month after stopping treatment. Do not administer VERQUVO to a pregnant female because it may cause fetal harm [see Dosage and Administration (2.2), Warnings and Precautions (5.1), and Use in Specific Populations (8.3)].
-
1 INDICATIONS AND USAGE
VERQUVO® is indicated to reduce the risk of cardiovascular death and heart failure (HF) hospitalization following a hospitalization for heart failure or need for outpatient IV diuretics, in adults with symptomatic chronic HF and ejection fraction less than 45% [see Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended starting dose of VERQUVO is 2.5 mg orally once daily with food.
Double the dose of VERQUVO approximately every 2 weeks to reach the target maintenance dose of 10 mg once daily, as tolerated by the patient.
For patients who are unable to swallow whole tablets, VERQUVO may be crushed and mixed with water immediately before administration [see Clinical Pharmacology (12.3)].
2.2 Pregnancy Testing in Females of Reproductive Potential
Obtain a pregnancy test in females of reproductive potential prior to initiating treatment with VERQUVO [see Warnings and Precautions (5.1) and Use in Specific Populations (8.3)].
-
3 DOSAGE FORMS AND STRENGTHS
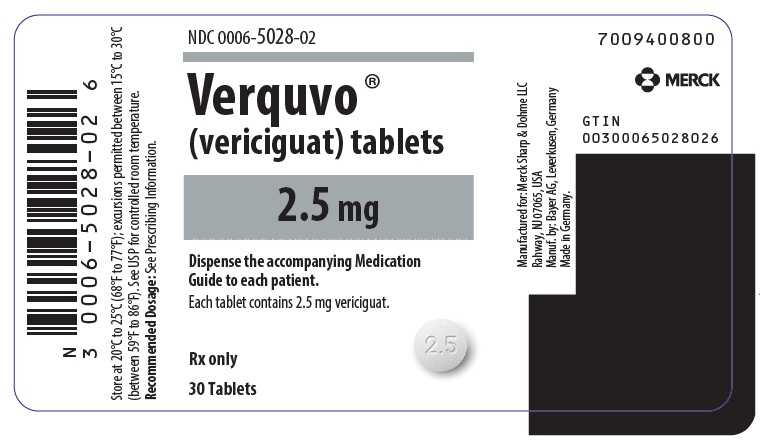
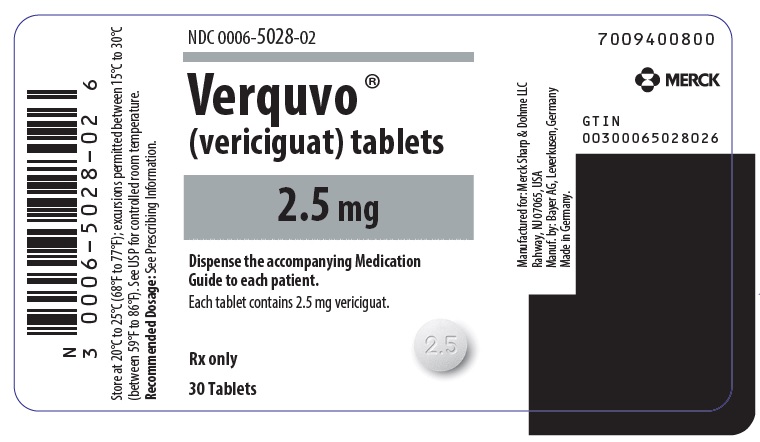
- VERQUVO 2.5 mg (vericiguat 2.5 mg) are round, biconvex, white film-coated tablets debossed with “2.5” on one side and “VC” on the other side.
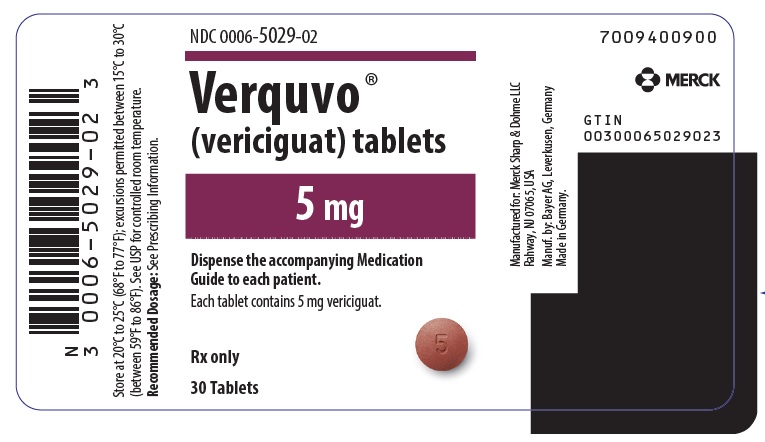
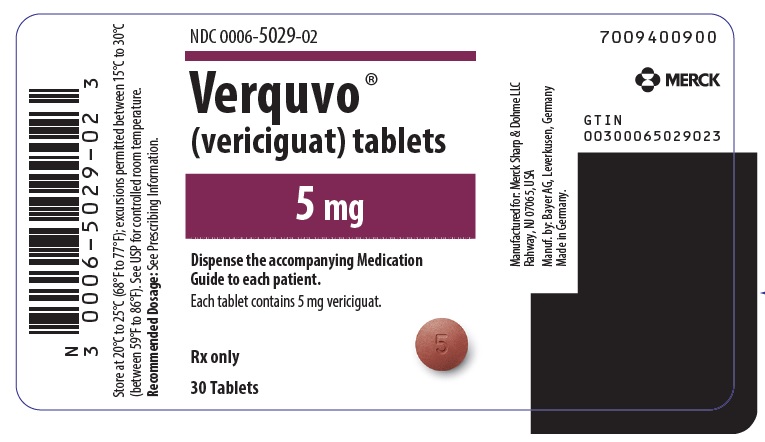
- VERQUVO 5 mg (vericiguat 5 mg) are round, biconvex, brown-red film-coated tablets debossed with “5” on one side and “VC” on the other side.
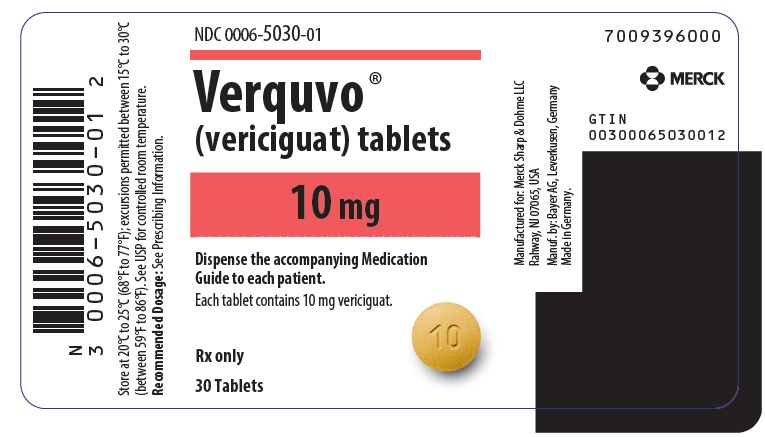
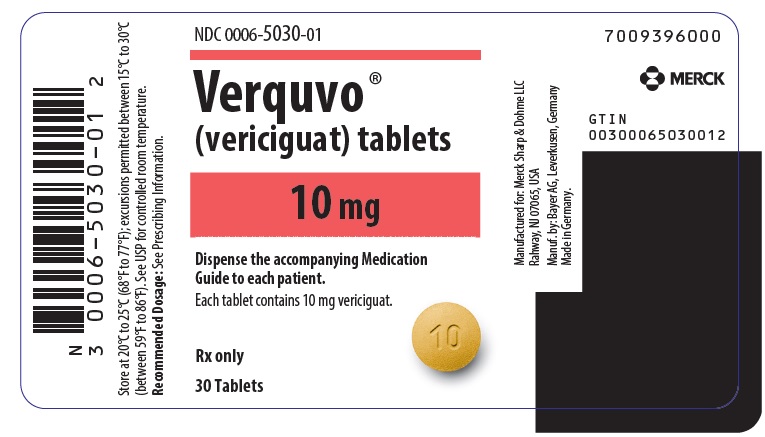
- VERQUVO 10 mg (vericiguat 10 mg) are round, biconvex, yellow-orange film-coated tablets debossed with “10” on one side and “VC” on the other side.
-
4 CONTRAINDICATIONS
VERQUVO is contraindicated in patients with concomitant use of other soluble guanylate cyclase (sGC) stimulators [see Drug Interactions (7.1)].
VERQUVO is contraindicated in pregnancy [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
Based on data from animal reproduction studies, VERQUVO may cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential of the potential risk to a fetus. Obtain a pregnancy test before the start of treatment. Advise females of reproductive potential to use effective contraception during treatment with VERQUVO and for at least one month after the final dose [see Dosage and Administration (2.2) and Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
VERQUVO was evaluated in VICTORIA, which included 2,519 patients treated with VERQUVO (up to 10 mg once daily). The mean duration of VERQUVO exposure was 1 year, and the maximum duration was 2.6 years [see Clinical Studies (14)]. Table 1 lists adverse drug reactions occurring more commonly with VERQUVO than placebo and in ≥5% of patients treated with VERQUVO in VICTORIA.
Table 1: Adverse Drug Reactions Occurring with VERQUVO in VICTORIA Adverse Drug Reaction VERQUVO %
N = 2,519Placebo %
N = 2,515Hypotension 16 15 Anemia 10 7 -
7 DRUG INTERACTIONS
7.1 Other Soluble Guanylate Cyclase Stimulators
VERQUVO is contraindicated in patients with concomitant use of other soluble guanylate cyclase (sGC) stimulators [see Contraindications (4)].
7.2 PDE-5 Inhibitors
Concomitant use of VERQUVO with PDE-5 inhibitors is not recommended because of the potential for hypotension [see Clinical Pharmacology (12.2)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Surveillance Program
There is a Pregnancy Surveillance Program that monitors pregnancy outcomes in women exposed to VERQUVO during pregnancy. Health care providers should report any prenatal exposure to VERQUVO by calling 1-877-888-4231 or at https://pregnancyreporting.verquvo-us.com.
Risk Summary
Based on data from animal reproduction studies, VERQUVO may cause fetal harm when administered to a pregnant woman and is contraindicated during pregnancy [see Contraindications (4)]. There are no available data with VERQUVO use in pregnant women. In animal reproduction studies, oral administration of vericiguat to pregnant rabbits during organogenesis, at ≥4 times the human exposure (total AUC) with the maximum recommended human dose (MRHD) of 10 mg, resulted in malformations of the heart and major vessels, as well as increased number of abortions and resorptions (see Animal Data). In a pre/postnatal toxicity study, vericiguat administered orally to rats during gestation through lactation caused maternal toxicity, which resulted in decreased pup body weight gain (≥10 times the MRHD) and increased pup mortality (24 times the MRHD) during the preweaning period (see Animal Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
In an embryo-fetal development study in rabbits, vericiguat was administered orally to pregnant rabbits during the period of organogenesis from gestation day (GD) 6 to 20 at doses of 0.75, 2.5 or 7.5 mg/kg/day. An increased incidence of cardiac ventricular septal defect along with truncus arteriosus communis was observed at ≥2.5 mg/kg/day, which is ≥4 times the human exposure at the MRHD. Maternal toxicity (decreased food consumption and body weight loss), which may have resulted in late spontaneous abortions and resorptions was noted at ≥2.5 mg/kg/day (≥4 times the human exposure at the MRHD). There were no maternal toxicity or abortions/resorptions and no malformations of the heart and major vessels in rabbits at an exposure approximately equivalent to the human exposure at the MRHD.
In a prenatal developmental toxicity study in rats, vericiguat was administered orally to pregnant rats during the period of organogenesis from GD 6 to 17 at doses of 5, 15 or 50 mg/kg/day. No developmental toxicity was observed up to the highest dose (36 times the human exposure [total AUC] at the MRHD). Maternal toxicity (decreased body weight gain and food consumption) was observed at ≥15 mg/kg/day (≥10 times the human exposure at the MRHD). There was no maternal toxicity at 5 mg/kg/day (4 times the human exposure at the MRHD).
In a pre-postnatal development study in rats, vericiguat was administered orally at doses of 7.5, 15 or 30 mg/kg/day from GD 6 through lactation day 21. Maternal toxicity (decreases in food consumption and body weight gain) was observed at all dose levels ≥6 times the human exposure (total AUC) at the MRHD and resulted in decreased pup body weight gain at ≥15 mg/kg/day (≥10 times the human exposure at the MRHD) and pup mortality at 30 mg/kg/day (24 times the MRHD).
[14C]-vericiguat was administered orally to pregnant rats at a dose of 3 mg/kg. Vericiguat-related material was transferred across the placenta, with fetal plasma concentrations of approximately 67% maternal concentrations on GD 19.
8.2 Lactation
Risk Summary
There are no data on the presence of vericiguat in human milk, the effects on the breastfed infant, or the effects on milk production. Vericiguat is present in the milk of lactating rats and it is likely that vericiguat or its metabolites are present in human milk (see Data). Because of the potential for serious adverse reactions in breastfed infants from VERQUVO, advise women not to breastfeed during treatment with VERQUVO.
[14C]-vericiguat was administered intravenously to lactating rats at a dose of 1 mg/kg. Vericiguat-related material was excreted into milk at concentrations approximately 12% maternal plasma concentrations on LD 8.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify the pregnancy status in females of reproductive potential prior to initiating VERQUVO [see Dosage and Administration (2.2), Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
Contraception
Females
VERQUVO may cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential to use effective contraception during treatment and for one month after the final dose [see Warnings and Precautions (5.1)].
8.4 Pediatric Use
Safety and effectiveness of VERQUVO have not been established in pediatric patients.
8.5 Geriatric Use
No dosage adjustment of VERQUVO is required in geriatric patients. In VICTORIA, a total of 1,596 (63%) patients treated with VERQUVO were 65 years and older, and 783 (31%) patients treated with VERQUVO were 75 years and older. No overall differences in safety or efficacy of VERQUVO were observed between patients aged 65 years and older compared to younger patients, but greater sensitivity of some older individuals cannot be ruled out [see Clinical Pharmacology (12.3) and Clinical Studies (14)].
8.6 Renal Impairment
No dosage adjustment of VERQUVO is recommended in patients with estimated glomerular filtration rate (eGFR) ≥15 mL/min/1.73m2 who are not on dialysis. VERQUVO has not been studied in patients with eGFR <15 mL/min/1.73m2 at treatment initiation or on dialysis [see Clinical Pharmacology (12.3) and Clinical Studies (14)].
8.7 Hepatic Impairment
No dosage adjustment of VERQUVO is recommended in patients with mild or moderate hepatic impairment (e.g., Child-Pugh A or B). VERQUVO has not been studied in patients with severe hepatic impairment (e.g., Child-Pugh C) [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Limited data are available with regard to overdosage in human patients treated with VERQUVO. In VICTORIA, doses up to 10 mg have been studied. In a study of patients with preserved ejection fraction heart failure (left ventricular ejection fraction ≥45%), multiple doses of VERQUVO 15 mg have been studied and were generally well tolerated. In the event of an overdose, hypotension may result. Symptomatic treatment should be provided. VERQUVO is unlikely to be removed by hemodialysis because of high protein binding.
-
11 DESCRIPTION
VERQUVO tablets contains vericiguat, a soluble guanylate cyclase stimulator.
The chemical name of vericiguat is methyl {4,6-diamino-2-[5-fluoro-1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b] pyridin-3-yl] pyrimidin-5-yl} carbamate. The molecular formula is C19H16F2N8O2 and the molecular weight is 426.39 g/mol.
The chemical structure is:
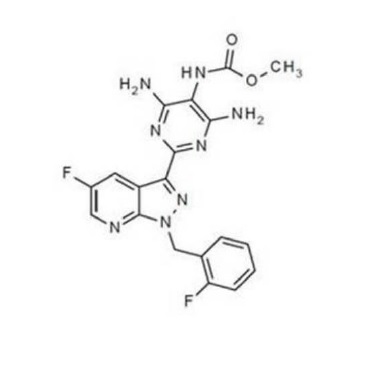
Vericiguat is a white to yellowish powder that is freely soluble in dimethyl sulfoxide; slightly soluble in acetone; very slightly soluble in ethanol, acetonitrile, methanol, and ethyl acetate; and practically insoluble in 2-propanol.
VERQUVO® is available as film-coated tablets for oral administration, containing 2.5 mg of vericiguat, 5 mg of vericiguat or 10 mg of vericiguat.
The tablet inactive ingredients are croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate.The film coating contains hypromellose, talc and titanium dioxide. The film coating for the 5 mg of VERQUVO tablet also contains ferric oxide red. The film coating for the 10 mg of VERQUVO tablet also contains ferric oxide yellow.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Vericiguat is a stimulator of soluble guanylate cyclase (sGC), an important enzyme in the nitric oxide (NO) signaling pathway. When NO binds to sGC, the enzyme catalyzes the synthesis of intracellular cyclic guanosine monophosphate (cGMP), a second messenger that plays a role in the regulation of vascular tone, cardiac contractility, and cardiac remodeling. Heart failure is associated with impaired synthesis of NO and decreased activity of sGC, which may contribute to myocardial and vascular dysfunction. By directly stimulating sGC, independently of and synergistically with NO, vericiguat augments levels of intracellular cGMP, leading to smooth muscle relaxation and vasodilation.
12.2 Pharmacodynamics
The mean reduction in systolic blood pressure was approximately 1 to 2 mm Hg greater in patients who received VERQUVO compared with placebo.
VERQUVO demonstrated a dose-dependent reduction in NT-proBNP, a biomarker in heart failure, at 12 weeks compared to placebo when added to standard of care. The estimated reduction from baseline NT-proBNP at week 32 was greater in patients who received VERQUVO compared with placebo [see Clinical Studies (14)].
Cardiac Electrophysiology
There was no evidence of proarrhythmic risk in an in vitro assessment of vericiguat or its major N-glucuronide metabolite. No inhibition of cardiac ion channels (hERG, hNav1.5, or hKvLQT1/mink) was observed at substantial multiples of their unbound Cmax values at the recommended target dose of 10 mg.The integrated risk assessment of nonclinical and clinical data supports that administration of vericiguat 10 mg is not associated with clinically meaningful QTc prolongation.
Drug Interaction Studies
No clinically significant differences on bleeding time or platelet aggregation were observed when a single dose of vericiguat 15 mg was used concomitantly with 500 mg of aspirin.No clinically significant differences in prothrombin time or the activities of Factors II, VII, and X were observed when multiple doses of VERQUVO 10 mg once daily were used concomitantly with a single dose of warfarin 25 mg.
No clinically significant differences on seated blood pressure (BP) were observed when multiple doses of VERQUVO 2.5 mg were used concomitantly with sacubitril/valsartan in healthy subjects.
No clinically significant differences on seated BP were observed when multiple doses of VERQUVO 10 mg were used concomitantly with short- and long-acting nitrates (nitroglycerin spray and isosorbide mononitrate [ISMN] modified release 60 mg) in patients with coronary artery disease. In patients with heart failure, concomitant use with short-acting nitrates was well tolerated, but there is limited experience with long-acting nitrates.
Concomitant use of VERQUVO 10 mg with single doses of sildenafil (25, 50, or 100 mg) was associated with additional seated BP reduction of up to 5.4 mm Hg (systolic/diastolic BP, MAP), compared to administration of VERQUVO alone. There is limited experience with concomitant use of VERQUVO and PDE-5 inhibitors in patients with heart failure.
12.3 Pharmacokinetics
Vericiguat steady-state mean (coefficient of variation %) Cmax is 350 mcg/L (29%) and AUC is 6,680 mcg•h/L (33.9%) following administration of VERQUVO 10 mg in patients with heart failure. Vericiguat pharmacokinetics increases in a slightly less than dose-proportional manner. Vericiguat accumulates in plasma up to 155-171% and reaches steady-state after approximately 6 days.
Absorption
The absolute bioavailability of vericiguat is 93% when taken with food. Results were comparable when VERQUVO was administered orally as a whole tablet or as a crushed tablet in water.Effect of Food
Administration of VERQUVO 10 mg with a high-fat, high-calorie meal increases Tmax from about 1 hour (fasted) to about 4 hours (fed), reduces PK variability, and increases vericiguat AUC by 44% and Cmax by 41% compared with administration in the fasted state. Similar results were obtained when VERQUVO was administered with a low-fat, low-calorie meal when compared to administration with a high-fat, high-calorie meal.Distribution
The mean steady-state volume of distribution of vericiguat is approximately 44 L in healthy subjects. Protein binding (primarily to serum albumin) of vericiguat is about 98%.Elimination
The half-life of vericiguat is 30 hours in patients with heart failure. Clearance in healthy subjects is 1.6 L/h.Metabolism
Vericiguat primarily undergoes glucuronidation by UGT1A9 and to a lesser extent, by UGT1A1 to form an inactive N-glucuronide metabolite. CYP-mediated metabolism is a minor clearance pathway (<5%).Excretion
Following oral administration of radiolabeled vericiguat to healthy subjects, approximately 53% of the dose was excreted in urine (primarily as inactive metabolite) and 45% in feces (primarily as unchanged drug).Specific Populations
Renal Impairment
In patients with heart failure with mild, moderate, and severe renal impairment not requiring dialysis, the mean exposure (AUC) of vericiguat was increased by 5%, 13%, and 20% respectively, compared to patients with normal renal function. These differences in exposure are not considered clinically relevant. The pharmacokinetics of vericiguat have not been studied in patients with eGFR <15 mL/min/1.73m2 at treatment initiation or on dialysis [see Use in Specific Populations (8.6)].In a dedicated clinical pharmacology study, otherwise healthy participants with mild, moderate, and severe renal impairment, had 8%, 73%, and 143% respectively, higher mean vericiguat exposure (unbound AUC normalized for body weight) after a single dose compared to healthy controls.
The apparent discrepancy of the effect of renal impairment on vericiguat exposure between the dedicated clinical pharmacology study and the analysis in patients with heart failure may be attributed to differences in study design and size.
Hepatic Impairment
No clinically relevant increases in exposure (unbound AUC normalized for body weight) were observed for individuals with mild and moderate hepatic impairment (Child Pugh A-B). Mean vericiguat exposures were 21% and 47% higher, respectively, compared to individuals with normal hepatic function. The pharmacokinetics of vericiguat have not been studied in patients with severe hepatic impairment (e.g., Child-Pugh C) [see Use in Specific Populations (8.7)].No clinically significant differences in the pharmacokinetics of vericiguat were observed based on age, sex, race/ethnicity (Black, White, Asian, Hispanic, Latino), body weight, or baseline NT-proBNP. Effects of specific populations on the pharmacokinetics of vericiguat are shown in Figure 1.
Figure 1: Pharmacokinetics of Vericiguat in Specific Populations 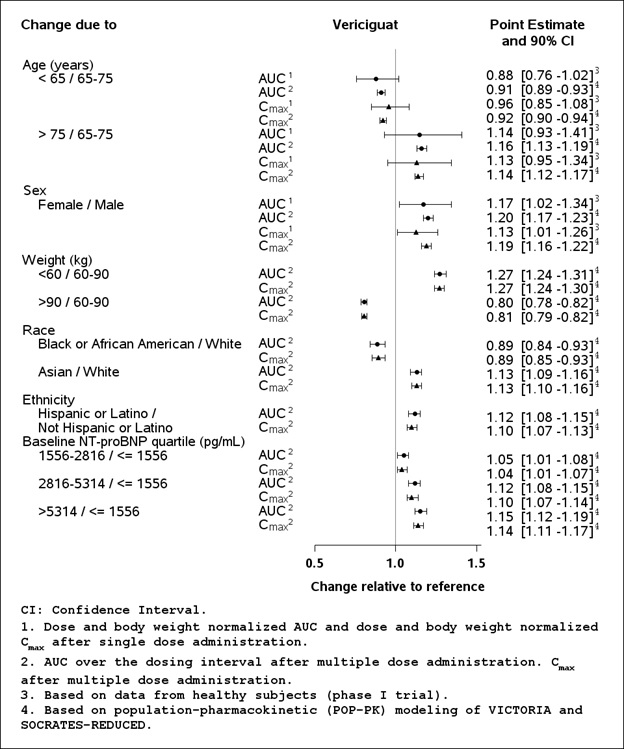
Drug Interaction Studies
Clinical Studies
Effects of Other Drugs on the Pharmacokinetics of Vericiguat
Vericiguat is less soluble at neutral than at acidic pH. Pre- and co-treatment with drugs that increase gastric pH, such as proton pump inhibitors or antacids, decrease vericiguat exposure (AUC) by about 30% following fasted administration. However, co-treatment with drugs that increase gastric pH did not affect vericiguat exposure in patients with heart failure when vericiguat was taken as directed with food.No clinically significant differences on vericiguat pharmacokinetics were observed with co-administration of mefenamic acid (UGT1A9 inhibitor), ketoconazole (multi-pathway CYP and transporter inhibitor), rifampin (inducer), digoxin (P-gp substrate), warfarin, aspirin, sildenafil, or the combination of sacubitril/valsartan in healthy subjects. No clinically significant differences on vericiguat pharmacokinetics were predicted with co-administration of atazanavir (UGT1A1 inhibitor).
Effects of Vericiguat on the Pharmacokinetics of Other Drugs
No clinically significant differences on the pharmacokinetics of midazolam (CYP3A substrate), digoxin (P-gp substrate), warfarin, sildenafil, or the combination of sacubitril (including metabolite LBQ657)/valsartan were observed when coadministered with VERQUVO in healthy subjects.In Vitro Studies
Cytochrome P450 (CYP) enzymes: vericiguat is not an inhibitor of CYP1A2, 2B6, 2C8, 2C9, 2C19, or 2D6, 3A4 and is not an inducer of CYP1A2, 2B6, or 3A4.Uridine diphosphate (UDP)-glucuronosyl transferase (UGT) enzymes: vericiguat is not an inhibitor of UGT1A1, 1A4, 1A6, 1A9, 2B4, or 2B7.
Transporter systems: vericiguat is a substrate of P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) but is not a substrate of organic cation transporter (OCT1) or organic anion transporting polypeptides (OATP1B1 and OATP1B3). Vericiguat is not an inhibitor of P-gp, BCRP, BSEP, OATP1B1/1B3, OAT1, OAT3, OCT1, OCT2, MATE1, or MATE2K.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity was evaluated in 2-year studies conducted in CD1 mice and Wistar rats. Vericiguat did not show a carcinogenic effect in mice dosed up to 150 mg/kg/day (males) or up to 250 mg/kg/day (females). These doses were associated with exposures 41 times (males) or 78 (females) times the human exposure (total AUC) at the MRHD of 10 mg/day.In the carcinogenicity study in rats, no vericiguat-related tumor or hyperplastic findings were observed at doses up to 20 mg/kg/day, at exposures of 16 (males) and 21 times (females) the human exposure at the MRHD.
Mutagenesis
Vericiguat was not genotoxic in the in vitro microbial mutagenicity (Ames) assay, the in vitro mouse lymphoma assay and the in vivo rat and mouse micronucleus assay.Impairment of Fertility
There were no effects on fertility, mating performance or early embryonic development when vericiguat was administered to rats at up to 32 times the human exposure (total AUC) at the MRHD.13.2 Animal Toxicology and/or Pharmacology
In growing rats, reversible effects on bone formation were observed, consisting of hypertrophy of growth plate and hyperostosis and remodeling of metaphyseal and diaphyseal bone. These effects were not observed after chronic administration of vericiguat at up to 22X (adult male rats), 25X (adult female rats), and 2.4X (adult dogs) the human exposure (total AUC) at the MRHD [see Use in Specific Populations (8.4)].
-
14 CLINICAL STUDIES
VICTORIA was a randomized, parallel-group, placebo-controlled, double-blind, event-driven, multi-center trial comparing VERQUVO and placebo in 5,050 adult patients with symptomatic chronic heart failure (New York Heart Association [NYHA] class II-IV) and left ventricular ejection fraction (LVEF) less than 45% following a worsening heart failure event. A worsening heart failure event was defined as heart failure hospitalization within 6 months before randomization or use of outpatient IV diuretics for heart failure within 3 months before randomization.
Patients were randomized to receive VERQUVO 10 mg or matching placebo. VERQUVO was initiated at 2.5 mg once daily and increased at approximately 2 week intervals to 5 mg once daily and the target dose of 10 mg once daily, as tolerated. Placebo doses were similarly adjusted. After approximately 1 year, 90% of patients in both treatment groups were treated with the 10 mg target dose.
The primary endpoint was a composite of time to first event of CV death or hospitalization for heart failure. The median follow-up for the primary endpoint was 11 months.
The population was 64% Caucasian, 22% Asian, and 5% Black. The mean age was 67 years and 76% were male. At randomization, 59% of patients were NYHA Class II, 40% were NYHA Class III, and 1% were NYHA Class IV. The mean left ventricular ejection fraction (EF) was 29%. Approximately half of all patients had an EF <30%, and 14% had an EF between 40% and 45%. The most frequently reported medical history conditions other than heart failure included hypertension (79%), coronary artery disease (58%), hyperlipidemia (57%), diabetes mellitus (47%), atrial fibrillation (45%) and myocardial infarction (42%). At randomization, the mean eGFR was 62 mL/min/1.73 m2; the majority of patients (88%) had an eGFR >30 mL/min/1.73 m2. Sixty-seven percent of the patients were enrolled within 3 months of a HF-hospitalization index event; 17% were enrolled within 3 to 6 months of HF hospitalization, and 16% were enrolled within 3 months of outpatient treatment with IV diuretics for worsening HF. The median NT-proBNP level was 2800 pg/mL at randomization.
At baseline, 93% of patients were on a beta blocker, 73% of patients were on an angiotensin-converting enzyme (ACE) inhibitor or angiotensin II receptor blocker (ARB), 70% of patients were on a mineralocorticoid receptor antagonist (MRA), 15% of patients were on a combination of an angiotensin receptor and neprilysin inhibitor (ARNI), 28% of patients had an implantable cardiac defibrillator, and 15% had a biventricular pacemaker. Ninety-one percent of patients were treated with 2 or more heart failure medications (beta blocker, any renin-angiotensin system [RAS] inhibitor or MRA) and 60% of patients were treated with all 3. At baseline, 6% of patients were on ivabradine and 3% of patients were on a sodium glucose co-transporter 2 (SGLT2) inhibitor.
In VICTORIA, VERQUVO was superior to placebo in reducing the risk of CV death or heart failure hospitalization based on a time-to-event analysis (hazard ratio [HR]: 0.90, 95% confidence interval [CI], 0.82-0.98; p=0.019). Over the course of the study, there was a 4.2% annualized absolute risk reduction (ARR) with VERQUVO compared with placebo. The treatment effect reflected a reduction in both cardiovascular death and heart failure hospitalization (see Table 2).
Table 2: Treatment Effect for the Primary Composite Endpoint and the Secondary Endpoints of Cardiovascular Death and Heart Failure Hospitalization VERQUVO
N=2,526Placebo
N=2,524Treatment Comparison n (%) Event rate:
% of
patients
per year*n (%) Event rate:
% of
patients
per year*Hazard Ratio
(95% CI)†p-value‡ ARR§ N=Number of patients in Intent-to-Treat (ITT) population; n=Number of patients with an event. - *
- Total patients with an event per 100 patient years at risk.
- †
- Hazard ratio (VERQUVO over Placebo) and confidence interval from a Cox proportional hazards model.
- ‡
- From the log-rank test.
- §
- Absolute risk reduction, calculated as difference (Placebo-VERQUVO) in event rate per 100 patient years.
- ¶
- For patients with multiple events, only the first event contributing to the composite endpoint is counted.
Primary endpoint Composite of
cardiovascular death
or heart failure
hospitalization¶
897 (35.5) 33.6 972 (38.5) 37.8 0.90
(0.82, 0.98)0.019 4.2 Secondary endpoints Cardiovascular death 414 (16.4) 12.9 441 (17.5) 13.9 0.93
(0.81,1.06)Heart failure
hospitalization
691 (27.4) 25.9 747 (29.6) 29.1 0.90
(0.81,1.00)The Kaplan-Meier curve (Figure 2) shows time to first occurrence of the primary composite endpoint of CV death or heart failure hospitalization.
Figure 2: Kaplan-Meier Curve for the Primary Composite Endpoint 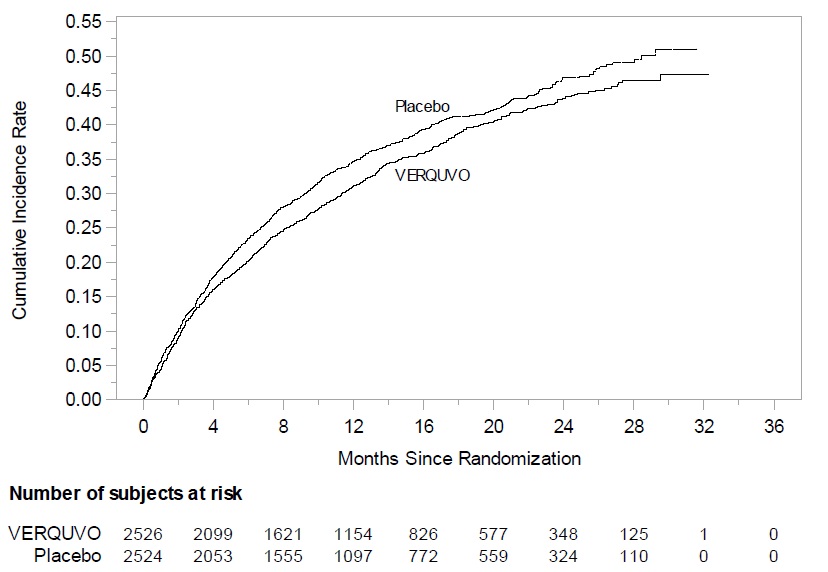
A wide range of demographic characteristics, baseline disease characteristics, and baseline concomitant medications were examined for their influence on outcomes. The results of the prespecified subgroup analysis for the primary composite endpoint are shown in Figure 3.
Figure 3: Primary Composite Endpoint (CV Death or HF Hospitalization) – Subgroup Analysis 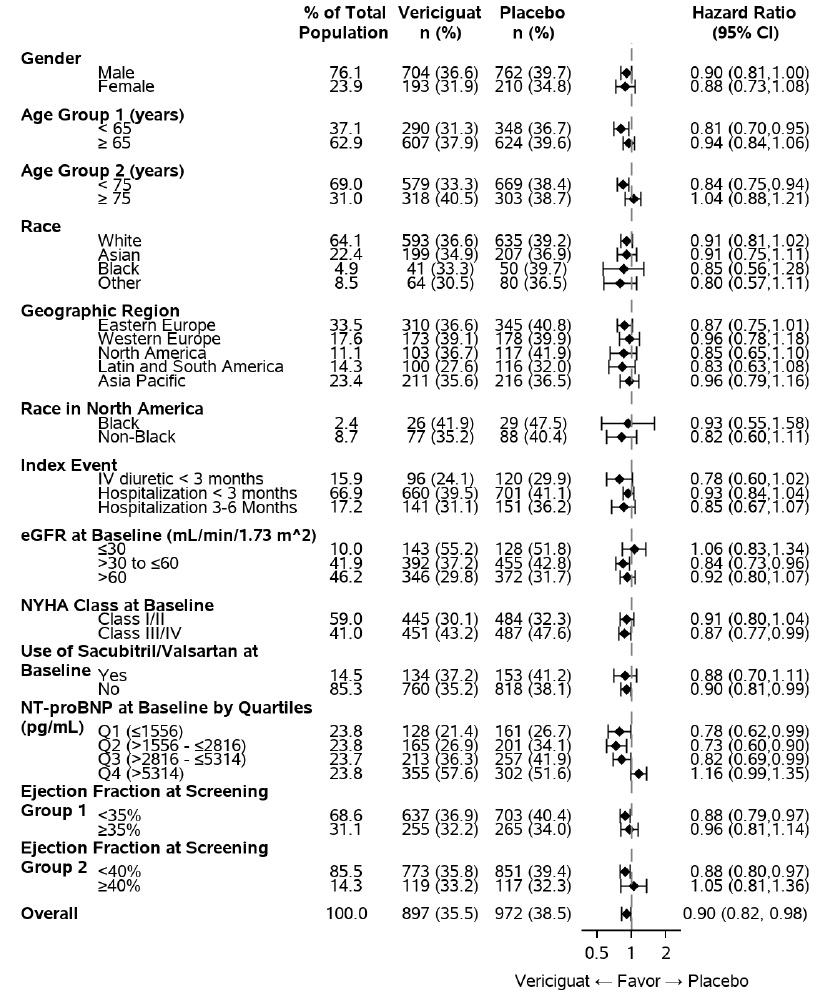
As shown above in Figure 3, the results of the primary composite endpoint were generally consistent across subgroups. However, among patients in the highest baseline NT-proBNP quartile, the estimated HRs for both CV death (HR: 1.16; 95% CI: [0.95, 1.43]) and first HF hospitalization (HR:1.19; 95%CI: [0.9,1.44]) were unfavorable, in contrast to the estimated HRs for patients in the three quartiles with lower NT-proBNP levels.
Secondary endpoints other than the components of the primary endpoint were tested according to a hierarchical testing procedure to control the family wise type I error rate. VERQUVO was superior to placebo in reducing the risk of total (first and recurrent) events of HF hospitalization and the first occurrence of either all-cause mortality or HF hospitalization (see Table 3).
Table 3: Treatment Effect for All-Cause Mortality or Heart Failure Hospitalization VERQUVO
N=2,526Placebo
N=2,524Hazard
Ratio
(95% CI)n (%) Rate n (%) Rate N=Number of patients in ITT population; n=Total number of events of heart failure hospitalization, or number of patients with ≥1 event for all other rows. - *
- Event rate (total events, including recurrent events in the same patient, per 100 patient years at risk).
- †
- Hazard ratio (VERQUVO over Placebo), based on an Andersen-Gill model.
- ‡
- For patients with multiple events, only the first event contributing to the composite endpoint is counted in this row and the applicable subsequent rows. Thus, any deaths occurring after a heart failure hospitalization are not counted.
- §
- Incidence rate (total patients with ≥1 event per 100 patient years at risk).
- ¶
- Hazard ratio (VERQUVO over Placebo), based on a Cox proportional hazards model.
Total events of heart
failure hospitalization
1,223 38.3* 1,336 42.4* 0.91†
(0.84, 0.99)Composite of all-
cause mortality or
heart failure
hospitalization‡
957 (37.9) 35.9§ 1,032 (40.9) 40.1§
0.90¶
(0.83, 0.98)- All-cause mortality 266 (10.5) 285 (11.3) - Heart failure
hospitalization691 (27.4) 747 (29.6) -
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
VERQUVO (vericiguat) is available as round, film-coated, biconvex tablets in the following configurations:
Strength Color Markings
(debossed)
Obverse/ReverseNDC # 14 Count
Bottle30 Count
Bottle90 Count
BottleCarton/100 * - *
- 10 blister cards of 10 tablets
2.5 mg White "2.5"/"VC" 0006-5028-01 0006-5028-02 - 0006-5028-04 5 mg Brown-Red "5"/"VC" 0006-5029-01 0006-5029-02 - 0006-5029-04 10 mg Yellow-Orange "10"/"VC" - 0006-5030-01 0006-5030-02 0006-5030-04 -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Dosing Instructions
If a dose is missed, it should be taken as soon as the patient remembers on the same day of the missed dose. Patients should not take two doses of VERQUVO on the same day.
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider of a known or suspected pregnancy. Advise females of reproductive potential to use effective contraception during treatment with VERQUVO and for one month after the final dose [see Contraindications (4), Warnings and Precautions (5.1) and Use in Specific Populations (8.1, 8.3)].Pregnancy
Advise women who are exposed to VERQUVO during pregnancy to report their pregnancy to their healthcare provider. Health care providers should report any prenatal exposure to VERQUVO by calling 1-877-888-4231 or at https://pregnancyreporting.verquvo-us.com. [See Use in Specific Populations (8.1)].Lactation
Advise women not to breastfeed during treatment with VERQUVO [see Use in Specific Populations (8.2)]. -
SPL UNCLASSIFIED SECTION
Manufactured for: Merck Sharp & Dohme LLC
Rahway, NJ 07065, USAFor patent information: www.msd.com/research/patent
Copyright © 2021-2023 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.uspi-mk1242-t-2307r004
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 07/2023 MEDICATION GUIDE
VERQUVO® (ver-KYU-voh)
(vericiguat)
tabletsWhat is the most important information I should know about VERQUVO?
VERQUVO may cause birth defects if taken during pregnancy.- Females must not be pregnant when they start taking VERQUVO.
- Females who are able to get pregnant:
- Your healthcare provider will do a pregnancy test to make sure that you are not pregnant before you start taking VERQUVO.
- You must use effective forms of birth control during treatment and for 1 month after you stop treatment with VERQUVO. Talk to your healthcare provider about forms of birth control that you may use to prevent pregnancy during treatment with VERQUVO.
- Tell your healthcare provider right away if you become pregnant or think you are pregnant during treatment with VERQUVO.
- There is a Pregnancy Surveillance Program that monitors pregnancy outcomes in women exposed to VERQUVO during pregnancy. Patients should report any exposure to VERQUVO during pregnancy by calling 1-877-888-4231 or at https://pregnancyreporting.verquvo-us.com.
What is VERQUVO?
VERQUVO is a prescription medicine used in adults who are having symptoms of their chronic (long-lasting) heart failure, who have had a recent hospitalization or the need to receive intravenous (IV) medicines and have an ejection fraction (amount of blood pumped with each heartbeat) of less than 45 percent:- to reduce the risk of dying and
- to reduce the need to be hospitalized
It is not known if VERQUVO is safe and effective in children.Do not take VERQUVO if you: - are taking another medicine called a soluble guanylate cyclase stimulator (sGC). Ask your healthcare provider if you are not sure if you are taking an sGC medicine.
- are pregnant. See “What is the most important information I should know about VERQUVO?”
Before you take VERQUVO, tell your healthcare provider about all your medical conditions, including if you: - are breastfeeding or plan to breastfeed. It is not known if VERQUVO passes into your breast milk. Do not breastfeed if you take VERQUVO. Talk with your healthcare provider about the best way to feed your baby if you take VERQUVO.
How should I take VERQUVO? - Take VERQUVO exactly as your healthcare provider tells you to.
- Take VERQUVO 1 time each day with food.
- Swallow VERQUVO tablets whole. If you are not able to swallow the tablet whole, you may crush VERQUVO tablets and mix with water right before taking your dose.
- Your healthcare provider may change your dose — when you first start taking VERQUVO to find the best dose for you and how well you tolerate VERQUVO.
- If you miss a dose, take the missed dose as soon as you remember on the same day of the missed dose. Do not take 2 doses of VERQUVO on the same day to make up for a missed dose.
- If you take too much VERQUVO, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of VERQUVO?
VERQUVO may cause serious side effects, including:
See “What is the most important information I should know about VERQUVO?”
The most common side effects of VERQUVO include:- low blood pressure
- low red blood cells (anemia)
How should I store VERQUVO? - Store VERQUVO at room temperature between 68°F to 77°F (20°C to 25°C).
General information about the safe and effective use of VERQUVO.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use VERQUVO for a condition for which it was not prescribed. Do not give VERQUVO to other people, even if they have the same symptoms you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about VERQUVO that is written for health professionals.What are the ingredients in VERQUVO?
Active ingredient: vericiguat.
Inactive ingredients: croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, sodium lauryl sulfate.
The tablet film coating contains: hypromellose, talc, titanium dioxide. The film-coating for the 5 mg tablet also contains ferric oxide red. The film-coating for the 10 mg tablet also contains ferric oxide yellow.Manufactured for: Merck Sharp & Dohme LLC
Rahway, NJ 07065, USA
For patent information: www.msd.com/research/patent
Copyright © 2021-2023 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.
usmg-mk1242-t-2307r004 - PRINCIPAL DISPLAY PANEL - 2.5 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 5 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 10 mg Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
VERQUVO
vericiguat tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0006-5028 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VERICIGUAT (UNII: LV66ADM269) (VERICIGUAT - UNII:LV66ADM269) VERICIGUAT 2.5 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score no score Shape ROUND Size 7mm Flavor Imprint Code 2;5;VC Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-5028-01 14 in 1 BOTTLE; Type 0: Not a Combination Product 02/01/2021 2 NDC:0006-5028-02 30 in 1 BOTTLE; Type 0: Not a Combination Product 02/01/2021 3 NDC:0006-5028-04 10 in 1 CARTON 02/01/2021 3 NDC:0006-5028-03 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 4 NDC:0006-5028-06 1 in 1 CARTON 04/01/2023 4 NDC:0006-5028-05 14 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214377 02/01/2021 VERQUVO
vericiguat tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0006-5029 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VERICIGUAT (UNII: LV66ADM269) (VERICIGUAT - UNII:LV66ADM269) VERICIGUAT 5 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color BROWN (brown-red) Score no score Shape ROUND Size 7mm Flavor Imprint Code 5;VC Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-5029-01 14 in 1 BOTTLE; Type 0: Not a Combination Product 02/01/2021 2 NDC:0006-5029-02 30 in 1 BOTTLE; Type 0: Not a Combination Product 02/01/2021 3 NDC:0006-5029-04 10 in 1 CARTON 02/01/2021 3 NDC:0006-5029-03 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 4 NDC:0006-5029-06 1 in 1 CARTON 02/01/2021 4 NDC:0006-5029-05 14 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214377 02/01/2021 VERQUVO
vericiguat tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0006-5030 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VERICIGUAT (UNII: LV66ADM269) (VERICIGUAT - UNII:LV66ADM269) VERICIGUAT 10 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color YELLOW (yellow-orange) Score no score Shape ROUND Size 9mm Flavor Imprint Code 10;VC Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-5030-01 30 in 1 BOTTLE; Type 0: Not a Combination Product 02/01/2021 2 NDC:0006-5030-02 90 in 1 BOTTLE; Type 0: Not a Combination Product 02/01/2021 3 NDC:0006-5030-04 10 in 1 CARTON 02/01/2021 3 NDC:0006-5030-03 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214377 02/01/2021 Labeler - Merck Sharp & Dohme LLC (118446553)