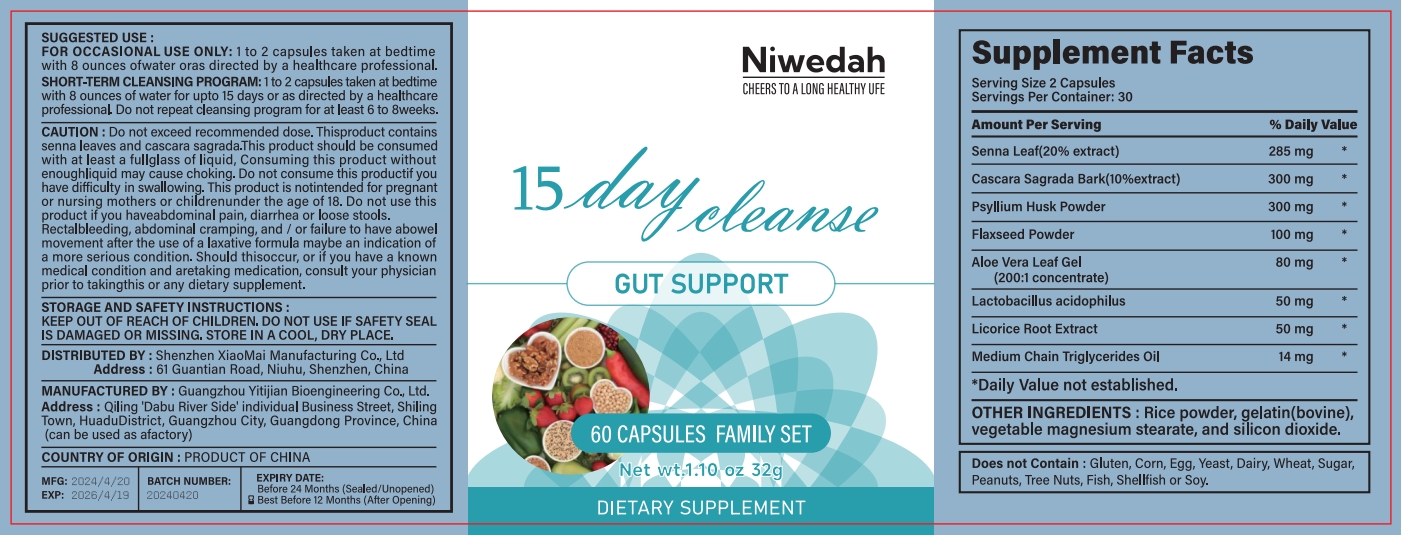
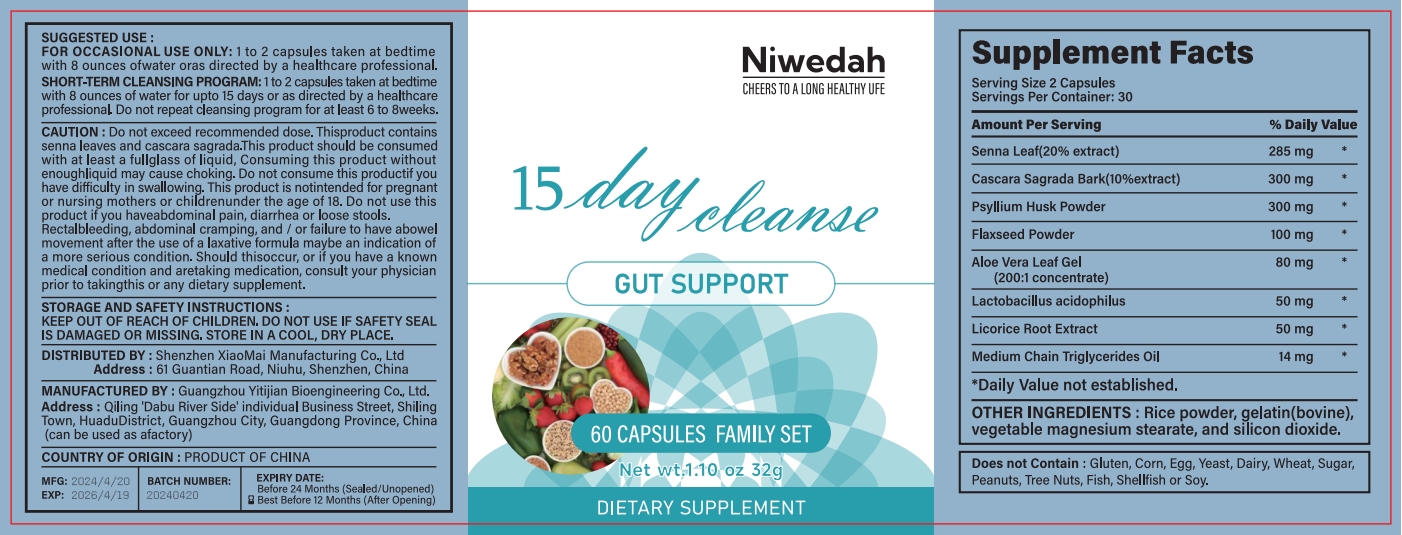
Label: NIWEDAH DAY CLEANSE GUT SUPPORT- day cleanse gut support capsule
- NDC Code(s): 83872-117-01
- Packager: Shenzhen XiaoMai Manufacturing Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- Uses
- WARNINGS
- Do not use
- When using this product
- Stop use and ask a doctor
- Keep out of reach of children.
- Directions for use
- INACTIVE INGREDIENT
- Other Information
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NIWEDAH DAY CLEANSE GUT SUPPORT
day cleanse gut support capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83872-117 Route of Administration INTRAGASTRIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNA LEAF (UNII: AK7JF626KX) (SENNA LEAF - UNII:AK7JF626KX) SENNA LEAF 17 mg in 1 g Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) 32 mg in 1 g Product Characteristics Color white Score score with uneven pieces Shape CAPSULE Size 20mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83872-117-01 113 g in 1 BOTTLE; Type 0: Not a Combination Product 04/25/2024 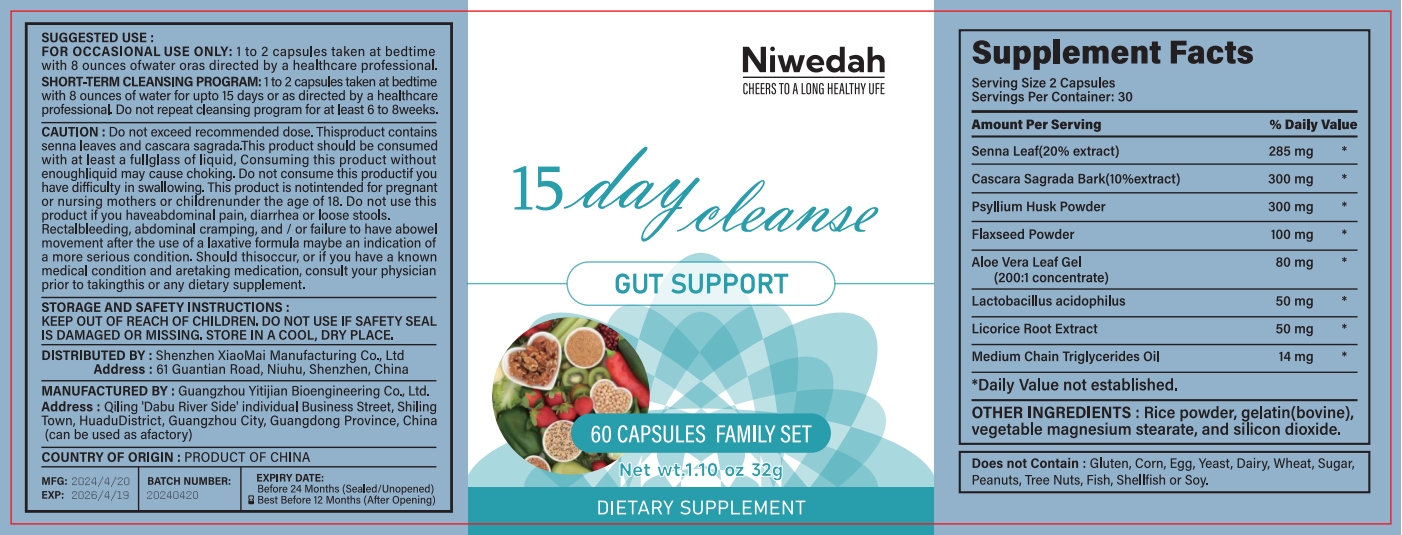
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 04/25/2024 Labeler - Shenzhen XiaoMai Manufacturing Co., Ltd. (712999147) Establishment Name Address ID/FEI Business Operations Shenzhen XiaoMai Manufacturing Co., Ltd. 712999147 manufacture(83872-117)