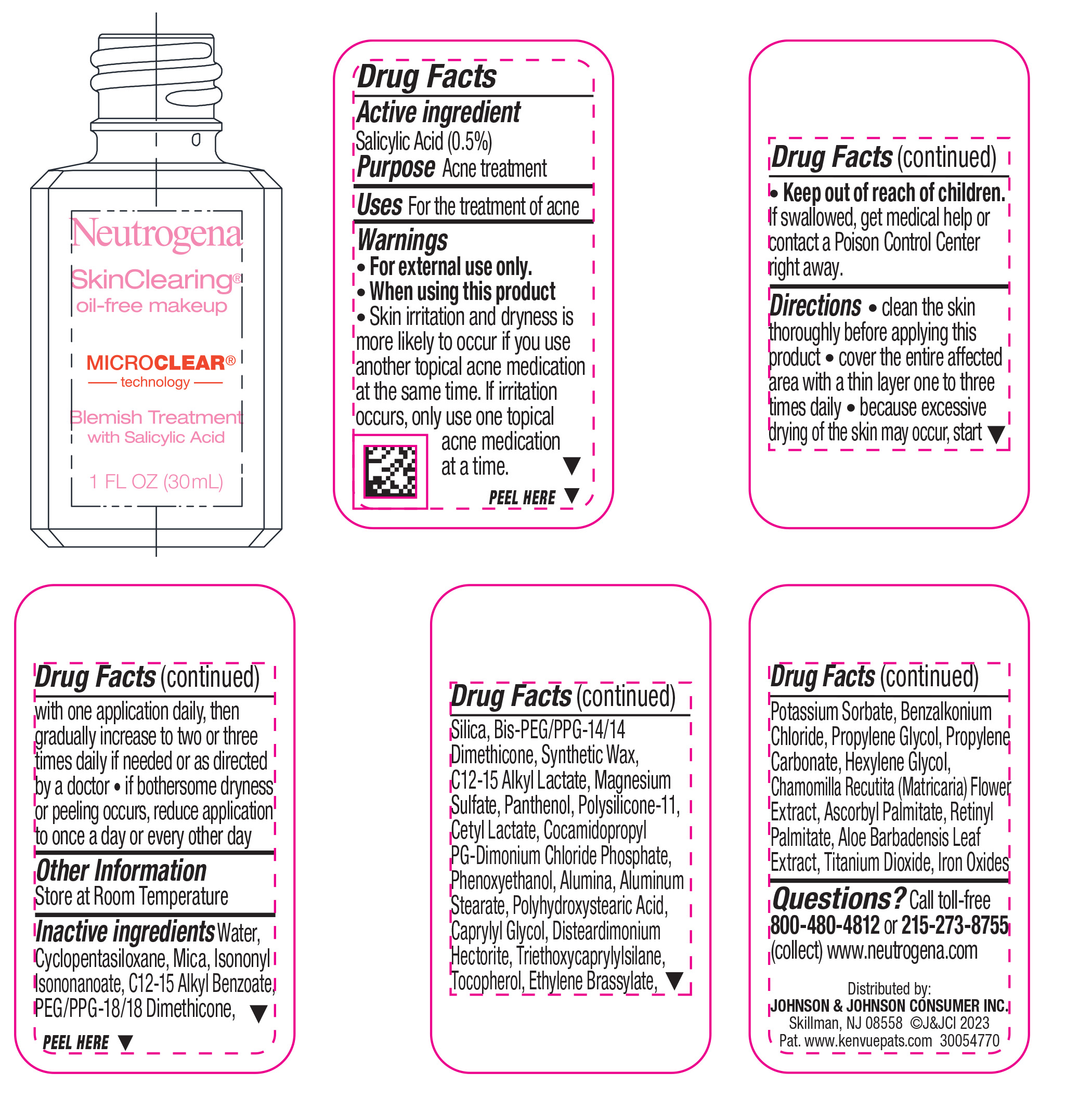
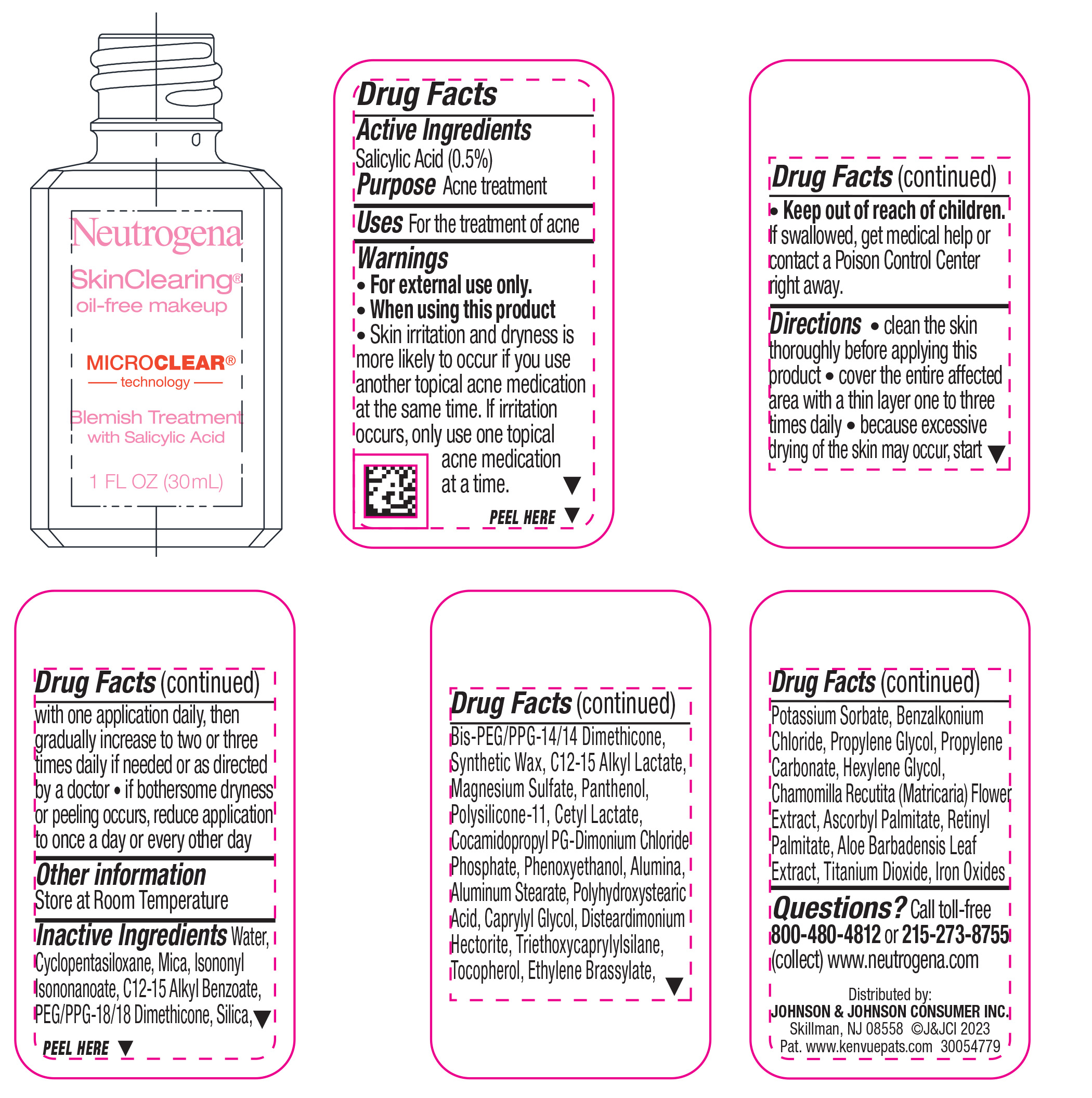
Label: NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - SOFT BEIGE 50- salicylic acid liquid
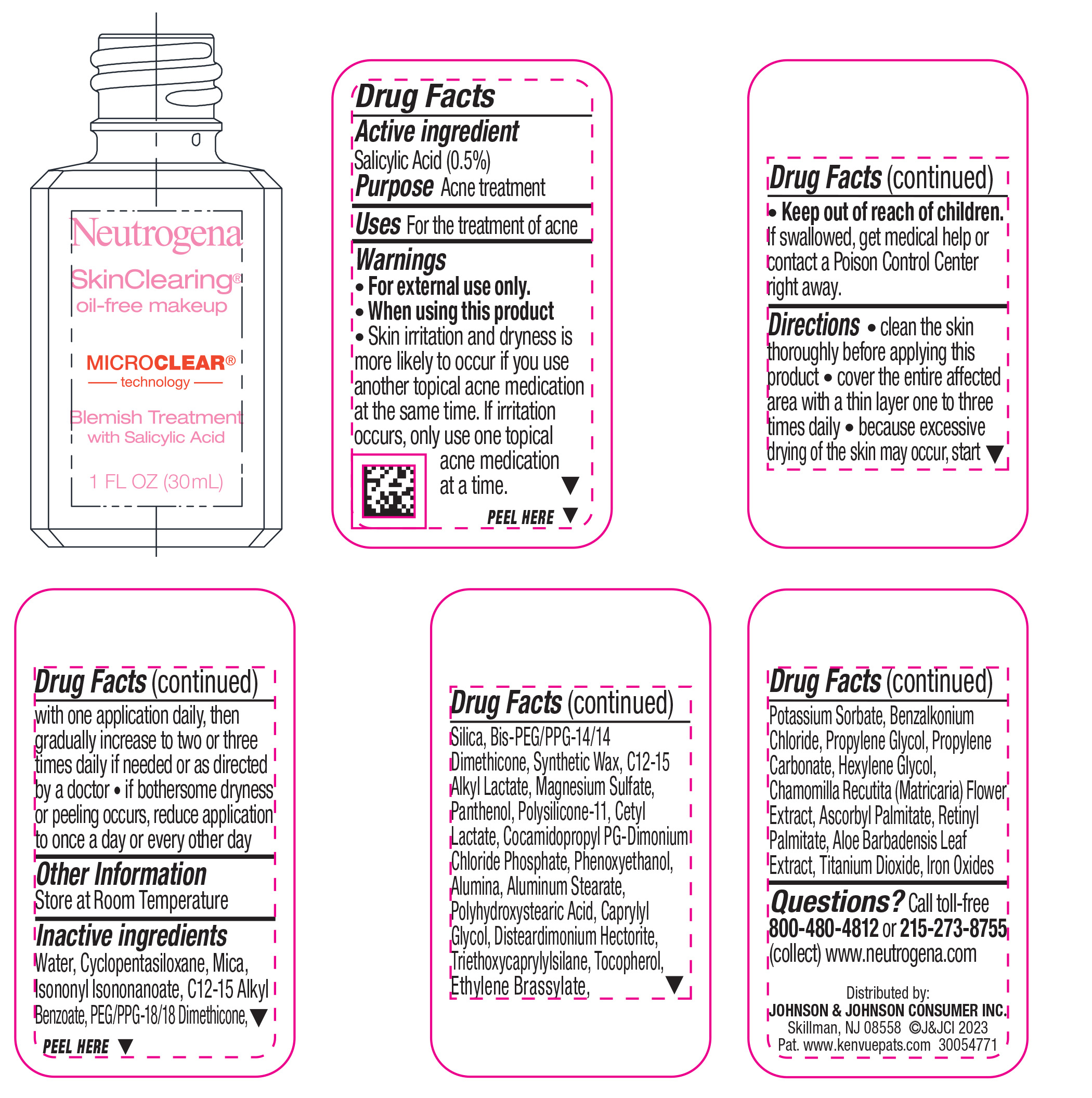
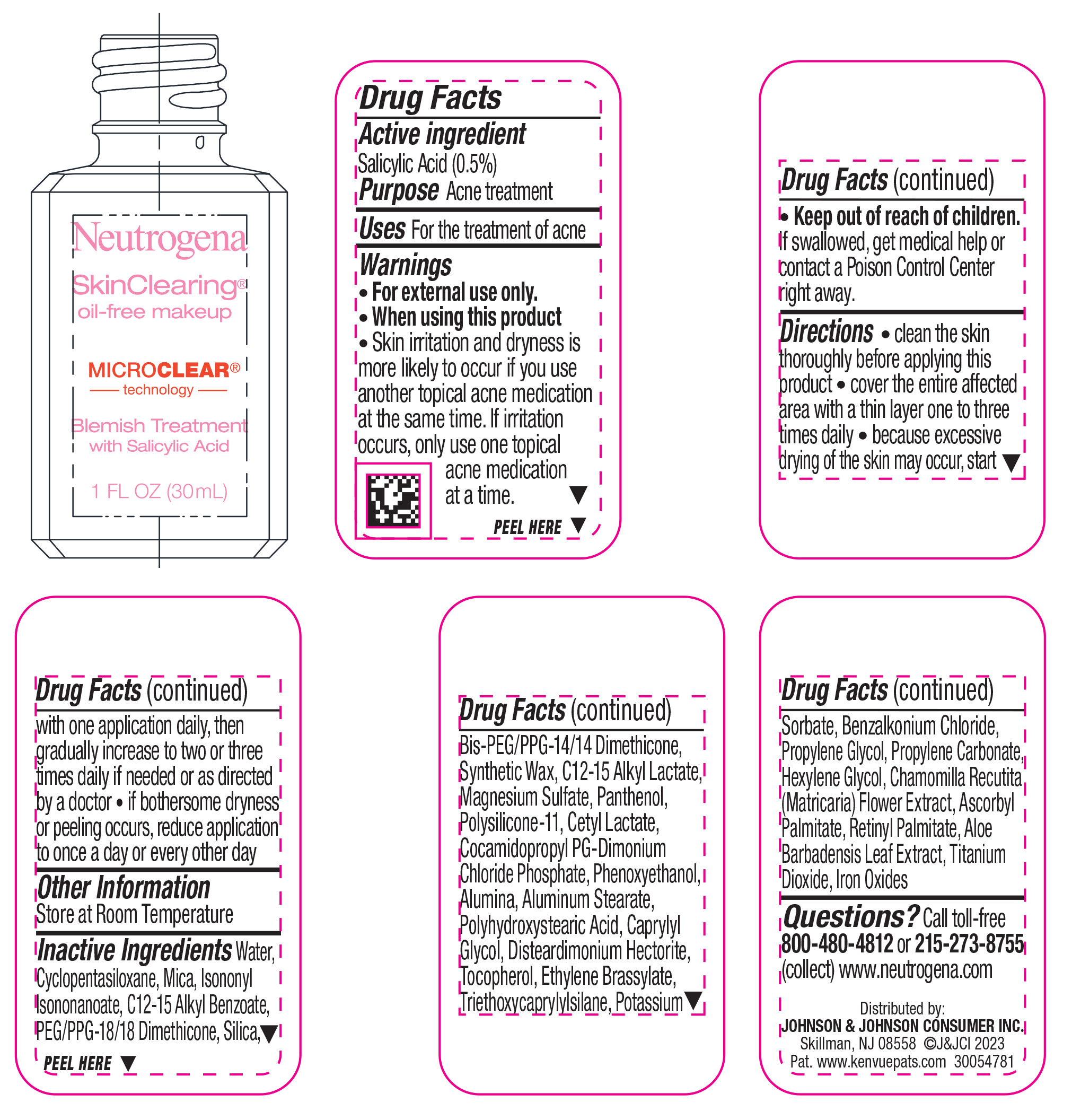
NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - WARM BEIGE 90- salicylic acid liquid
NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - BUFF 30- salicylic acid liquid
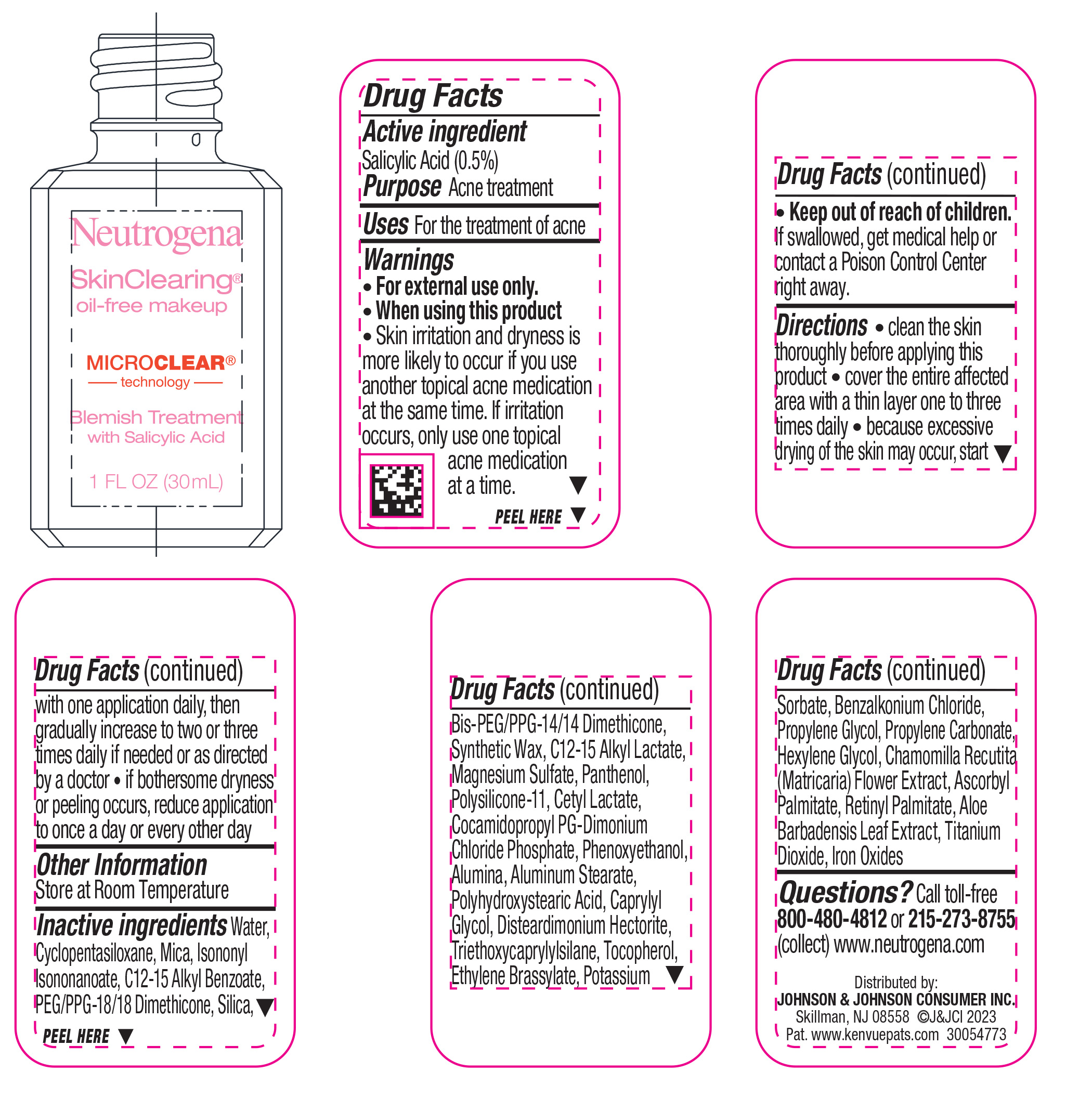
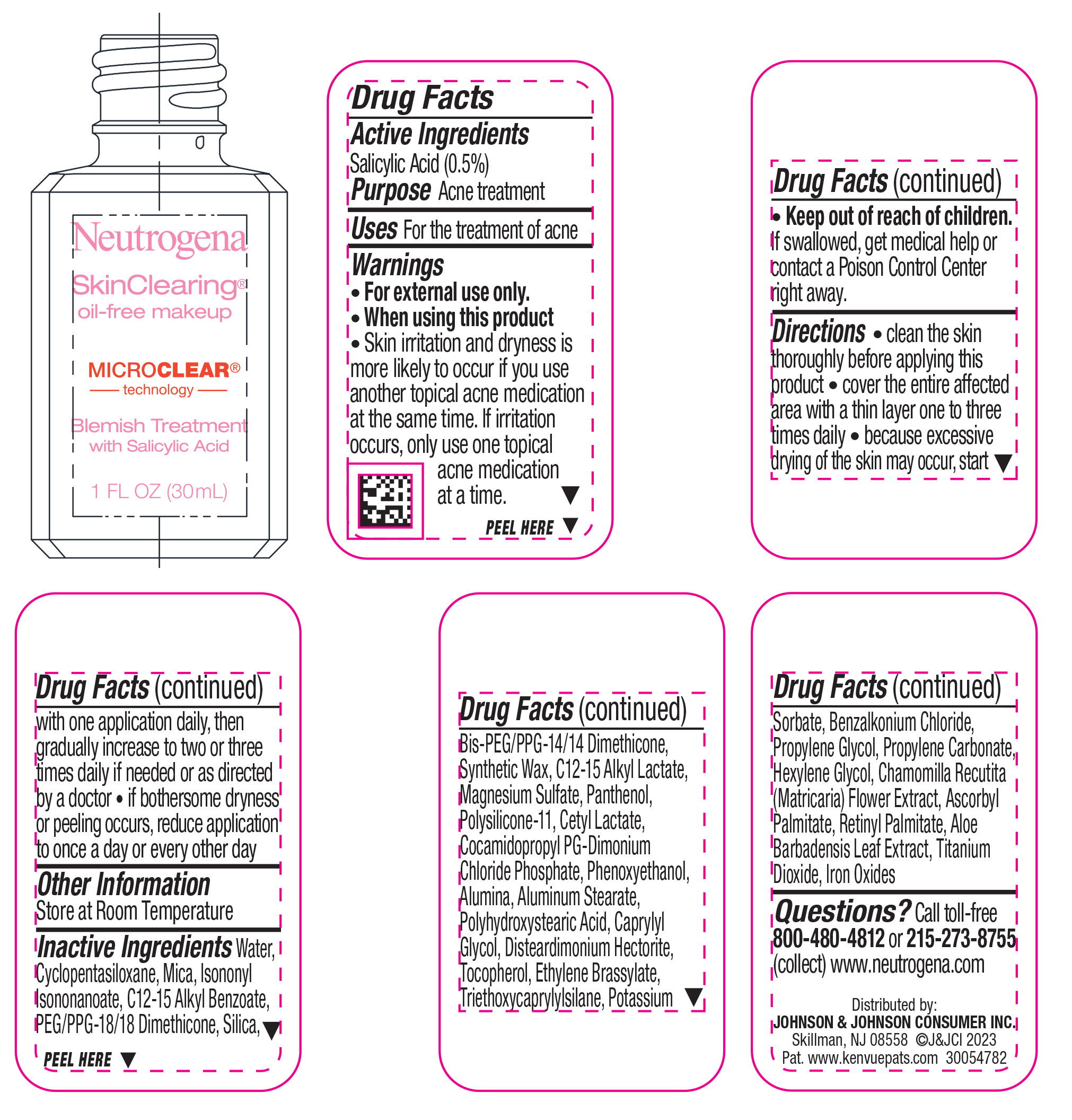
NEUTROGENA SKINCLEARING OIL-FREE MAKEUP- NATURAL TAN 100- salicylic acid liquid
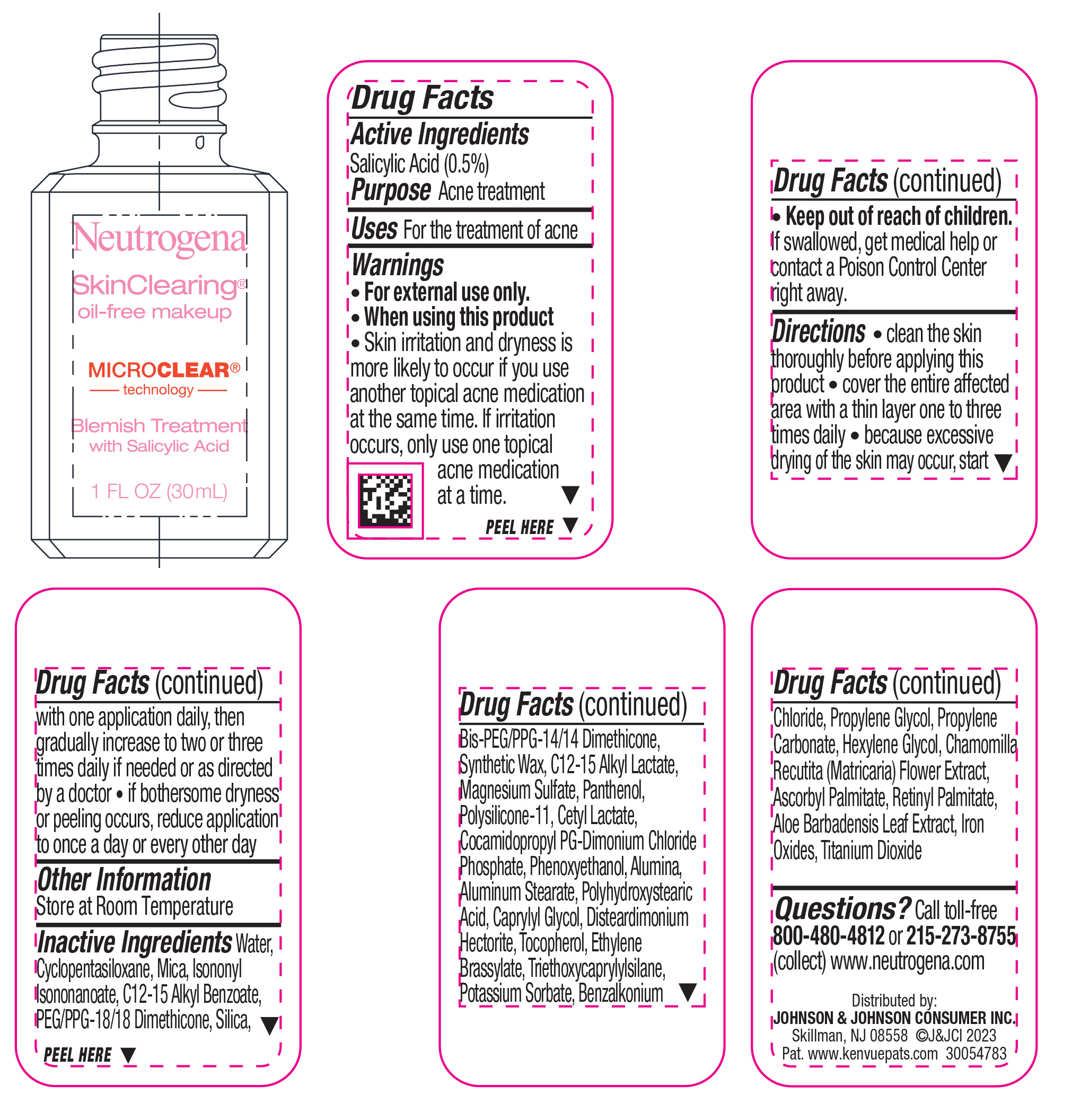
NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - FRESH BEIGE 70- salicylic acid liquid
NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - NATURAL BEIGE 60- salicylic acid liquid
NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - HONEY 85- salicylic acid liquid
NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - CLASSIC IVORY 10- salicylic acid liquid
NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - NUDE 40- salicylic acid liquid
NEUTROGENA SKINCLEARING OIL-FREE MAKEUP- MEDIUM BEIGE 80- salicylic acid liquid
NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - CARAMEL 105- salicylic acid liquid
NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - COCOA 115- salicylic acid liquid
NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - NATURAL IVORY 20- salicylic acid liquid
NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - CHESTNUT 135- salicylic acid liquid
-
NDC Code(s):
69968-0853-1,
69968-0854-1,
69968-0855-1,
69968-0856-1, view more69968-0857-1, 69968-0858-1, 69968-0859-1, 69968-0860-1, 69968-0861-1, 69968-0862-1, 69968-0863-1, 69968-0864-1, 69968-0865-1, 69968-0866-1
- Packager: Kenvue Brands LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
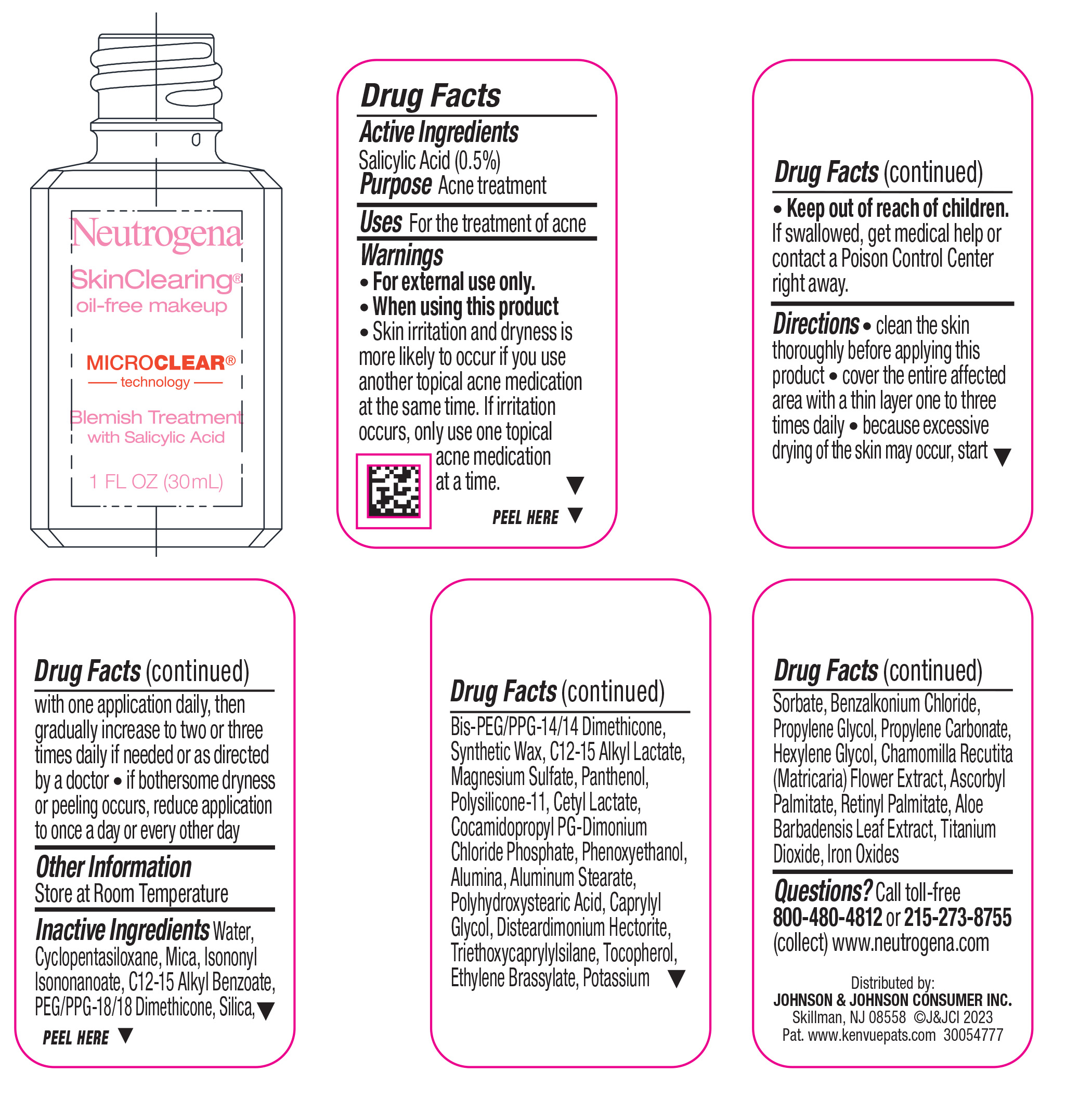
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
• clean the skin thoroughly before applying this product
• cover the entire affected area with a thin layer one to three times daily
• because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
• if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- Other Information
-
Inactive ingredients
Water, Cyclopentasiloxane, Mica, Isononyl Isononanoate, C12-15 Alkyl Benzoate, PEG/PPG-18/18 Dimethicone, Silica, Bis-PEG/PPG-14/14 Dimethicone, Synthetic Wax, C12-15 Alkyl Lactate, Magnesium Sulfate, Panthenol, Polysilicone-11, Cetyl Lactate, Cocamidopropyl PG-Dimonium Chloride Phosphate, Phenoxyethanol, Alumina, Aluminum Stearate, Polyhydroxystearic Acid, Caprylyl Glycol, Disteardimonium Hectorite, Tocopherol, Ethylene Brassylate, Triethoxycaprylylsilane, Potassium Sorbate, Benzalkonium Chloride, Propylene Glycol, Propylene Carbonate, Hexylene Glycol, Chamomilla Recutita (Matricaria) Flower Extract, Ascorbyl Palmitate, Retinyl Palmitate, Aloe Barbadensis Leaf Extract, Iron Oxides, Titanium Dioxide
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Classic Ivory 10
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Natural Ivory 20
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Buff 30
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Nude 40
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Soft Beige 50
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Natural Beige 60
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Fresh Beige 70
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Medium Beige 80
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Honey 85
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Warm Beige 90
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Natural Tan 100
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Caramel 105
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Cocoa 115
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Chestnut 135
-
INGREDIENTS AND APPEARANCE
NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - SOFT BEIGE 50
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0857 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) HEXYLENE GLYCOL (UNII: KEH0A3F75J) FERROUS OXIDE (UNII: G7036X8B5H) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) ALUMINUM OXIDE (UNII: LMI26O6933) ALUMINUM STEARATE (UNII: U6XF9NP8HM) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) CETYL LACTATE (UNII: A7EVH2RK4O) CHAMOMILE (UNII: FGL3685T2X) PROPYLENE CARBONATE (UNII: 8D08K3S51E) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ASCORBYL PALMITATE (UNII: QN83US2B0N) TOCOPHEROL (UNII: R0ZB2556P8) MICA (UNII: V8A1AW0880) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BIS-PEG/PPG-14/14 DIMETHICONE (UNII: X2I70H0QJE) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0857-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 06/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 06/14/2024 NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - WARM BEIGE 90
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0862 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) HEXYLENE GLYCOL (UNII: KEH0A3F75J) FERROUS OXIDE (UNII: G7036X8B5H) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) ALUMINUM OXIDE (UNII: LMI26O6933) ALUMINUM STEARATE (UNII: U6XF9NP8HM) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) CETYL LACTATE (UNII: A7EVH2RK4O) CHAMOMILE (UNII: FGL3685T2X) PROPYLENE CARBONATE (UNII: 8D08K3S51E) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ASCORBYL PALMITATE (UNII: QN83US2B0N) TOCOPHEROL (UNII: R0ZB2556P8) MICA (UNII: V8A1AW0880) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BIS-PEG/PPG-14/14 DIMETHICONE (UNII: X2I70H0QJE) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0862-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 06/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 06/14/2024 NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - BUFF 30
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0855 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) HEXYLENE GLYCOL (UNII: KEH0A3F75J) FERROUS OXIDE (UNII: G7036X8B5H) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) ALUMINUM OXIDE (UNII: LMI26O6933) ALUMINUM STEARATE (UNII: U6XF9NP8HM) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) CETYL LACTATE (UNII: A7EVH2RK4O) CHAMOMILE (UNII: FGL3685T2X) PROPYLENE CARBONATE (UNII: 8D08K3S51E) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ASCORBYL PALMITATE (UNII: QN83US2B0N) TOCOPHEROL (UNII: R0ZB2556P8) MICA (UNII: V8A1AW0880) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BIS-PEG/PPG-14/14 DIMETHICONE (UNII: X2I70H0QJE) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0855-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 06/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 06/14/2024 NEUTROGENA SKINCLEARING OIL-FREE MAKEUP- NATURAL TAN 100
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0863 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) HEXYLENE GLYCOL (UNII: KEH0A3F75J) FERROUS OXIDE (UNII: G7036X8B5H) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) ALUMINUM OXIDE (UNII: LMI26O6933) ALUMINUM STEARATE (UNII: U6XF9NP8HM) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) CETYL LACTATE (UNII: A7EVH2RK4O) CHAMOMILE (UNII: FGL3685T2X) PROPYLENE CARBONATE (UNII: 8D08K3S51E) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ASCORBYL PALMITATE (UNII: QN83US2B0N) TOCOPHEROL (UNII: R0ZB2556P8) MICA (UNII: V8A1AW0880) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BIS-PEG/PPG-14/14 DIMETHICONE (UNII: X2I70H0QJE) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0863-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 06/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 06/14/2024 NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - FRESH BEIGE 70
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0859 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) HEXYLENE GLYCOL (UNII: KEH0A3F75J) FERROUS OXIDE (UNII: G7036X8B5H) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) ALUMINUM OXIDE (UNII: LMI26O6933) ALUMINUM STEARATE (UNII: U6XF9NP8HM) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) CETYL LACTATE (UNII: A7EVH2RK4O) CHAMOMILE (UNII: FGL3685T2X) PROPYLENE CARBONATE (UNII: 8D08K3S51E) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ASCORBYL PALMITATE (UNII: QN83US2B0N) TOCOPHEROL (UNII: R0ZB2556P8) MICA (UNII: V8A1AW0880) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BIS-PEG/PPG-14/14 DIMETHICONE (UNII: X2I70H0QJE) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0859-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 06/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 06/14/2024 NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - NATURAL BEIGE 60
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0858 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) HEXYLENE GLYCOL (UNII: KEH0A3F75J) FERROUS OXIDE (UNII: G7036X8B5H) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) ALUMINUM OXIDE (UNII: LMI26O6933) ALUMINUM STEARATE (UNII: U6XF9NP8HM) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) CETYL LACTATE (UNII: A7EVH2RK4O) CHAMOMILE (UNII: FGL3685T2X) PROPYLENE CARBONATE (UNII: 8D08K3S51E) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ASCORBYL PALMITATE (UNII: QN83US2B0N) TOCOPHEROL (UNII: R0ZB2556P8) MICA (UNII: V8A1AW0880) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BIS-PEG/PPG-14/14 DIMETHICONE (UNII: X2I70H0QJE) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0858-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 06/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 06/14/2024 NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - HONEY 85
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0861 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) HEXYLENE GLYCOL (UNII: KEH0A3F75J) FERROUS OXIDE (UNII: G7036X8B5H) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) ALUMINUM OXIDE (UNII: LMI26O6933) ALUMINUM STEARATE (UNII: U6XF9NP8HM) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) CETYL LACTATE (UNII: A7EVH2RK4O) CHAMOMILE (UNII: FGL3685T2X) PROPYLENE CARBONATE (UNII: 8D08K3S51E) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ASCORBYL PALMITATE (UNII: QN83US2B0N) TOCOPHEROL (UNII: R0ZB2556P8) MICA (UNII: V8A1AW0880) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BIS-PEG/PPG-14/14 DIMETHICONE (UNII: X2I70H0QJE) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0861-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 06/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 06/14/2024 NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - CLASSIC IVORY 10
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0853 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) HEXYLENE GLYCOL (UNII: KEH0A3F75J) FERROUS OXIDE (UNII: G7036X8B5H) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) ALUMINUM OXIDE (UNII: LMI26O6933) ALUMINUM STEARATE (UNII: U6XF9NP8HM) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) MICA (UNII: V8A1AW0880) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BIS-PEG/PPG-14/14 DIMETHICONE (UNII: X2I70H0QJE) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) CETYL LACTATE (UNII: A7EVH2RK4O) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CHAMOMILE (UNII: FGL3685T2X) PROPYLENE CARBONATE (UNII: 8D08K3S51E) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ASCORBYL PALMITATE (UNII: QN83US2B0N) WATER (UNII: 059QF0KO0R) PANTHENOL (UNII: WV9CM0O67Z) TOCOPHEROL (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0853-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 06/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 06/14/2024 NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - NUDE 40
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0856 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) HEXYLENE GLYCOL (UNII: KEH0A3F75J) FERROUS OXIDE (UNII: G7036X8B5H) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) ALUMINUM OXIDE (UNII: LMI26O6933) ALUMINUM STEARATE (UNII: U6XF9NP8HM) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) CETYL LACTATE (UNII: A7EVH2RK4O) CHAMOMILE (UNII: FGL3685T2X) PROPYLENE CARBONATE (UNII: 8D08K3S51E) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ASCORBYL PALMITATE (UNII: QN83US2B0N) TOCOPHEROL (UNII: R0ZB2556P8) MICA (UNII: V8A1AW0880) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BIS-PEG/PPG-14/14 DIMETHICONE (UNII: X2I70H0QJE) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0856-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 06/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 06/14/2024 NEUTROGENA SKINCLEARING OIL-FREE MAKEUP- MEDIUM BEIGE 80
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0860 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) HEXYLENE GLYCOL (UNII: KEH0A3F75J) FERROUS OXIDE (UNII: G7036X8B5H) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) ALUMINUM OXIDE (UNII: LMI26O6933) ALUMINUM STEARATE (UNII: U6XF9NP8HM) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) CETYL LACTATE (UNII: A7EVH2RK4O) CHAMOMILE (UNII: FGL3685T2X) PROPYLENE CARBONATE (UNII: 8D08K3S51E) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ASCORBYL PALMITATE (UNII: QN83US2B0N) TOCOPHEROL (UNII: R0ZB2556P8) MICA (UNII: V8A1AW0880) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BIS-PEG/PPG-14/14 DIMETHICONE (UNII: X2I70H0QJE) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0860-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 06/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 06/14/2024 NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - CARAMEL 105
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0864 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) HEXYLENE GLYCOL (UNII: KEH0A3F75J) FERROUS OXIDE (UNII: G7036X8B5H) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) ALUMINUM OXIDE (UNII: LMI26O6933) ALUMINUM STEARATE (UNII: U6XF9NP8HM) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) CETYL LACTATE (UNII: A7EVH2RK4O) CHAMOMILE (UNII: FGL3685T2X) PROPYLENE CARBONATE (UNII: 8D08K3S51E) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ASCORBYL PALMITATE (UNII: QN83US2B0N) TOCOPHEROL (UNII: R0ZB2556P8) MICA (UNII: V8A1AW0880) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BIS-PEG/PPG-14/14 DIMETHICONE (UNII: X2I70H0QJE) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0864-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 06/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 06/14/2024 NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - COCOA 115
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0865 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) HEXYLENE GLYCOL (UNII: KEH0A3F75J) FERROUS OXIDE (UNII: G7036X8B5H) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) ALUMINUM OXIDE (UNII: LMI26O6933) ALUMINUM STEARATE (UNII: U6XF9NP8HM) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) CETYL LACTATE (UNII: A7EVH2RK4O) CHAMOMILE (UNII: FGL3685T2X) PROPYLENE CARBONATE (UNII: 8D08K3S51E) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ASCORBYL PALMITATE (UNII: QN83US2B0N) TOCOPHEROL (UNII: R0ZB2556P8) MICA (UNII: V8A1AW0880) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BIS-PEG/PPG-14/14 DIMETHICONE (UNII: X2I70H0QJE) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0865-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 06/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 06/14/2024 NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - NATURAL IVORY 20
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0854 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) HEXYLENE GLYCOL (UNII: KEH0A3F75J) FERROUS OXIDE (UNII: G7036X8B5H) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) ALUMINUM OXIDE (UNII: LMI26O6933) ALUMINUM STEARATE (UNII: U6XF9NP8HM) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) CETYL LACTATE (UNII: A7EVH2RK4O) CHAMOMILE (UNII: FGL3685T2X) PROPYLENE CARBONATE (UNII: 8D08K3S51E) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ASCORBYL PALMITATE (UNII: QN83US2B0N) TOCOPHEROL (UNII: R0ZB2556P8) MICA (UNII: V8A1AW0880) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BIS-PEG/PPG-14/14 DIMETHICONE (UNII: X2I70H0QJE) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0854-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 06/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 06/14/2024 NEUTROGENA SKINCLEARING OIL-FREE MAKEUP - CHESTNUT 135
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0866 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) HEXYLENE GLYCOL (UNII: KEH0A3F75J) FERROUS OXIDE (UNII: G7036X8B5H) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) ALUMINUM OXIDE (UNII: LMI26O6933) ALUMINUM STEARATE (UNII: U6XF9NP8HM) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) CETYL LACTATE (UNII: A7EVH2RK4O) CHAMOMILE (UNII: FGL3685T2X) PROPYLENE CARBONATE (UNII: 8D08K3S51E) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ASCORBYL PALMITATE (UNII: QN83US2B0N) TOCOPHEROL (UNII: R0ZB2556P8) MICA (UNII: V8A1AW0880) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BIS-PEG/PPG-14/14 DIMETHICONE (UNII: X2I70H0QJE) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0866-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 06/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 06/14/2024 Labeler - Kenvue Brands LLC (118772437)