Label: LEADER CHILDRENS MULTI SYMPTOM COLD- dextromethorphan hbr, guaifenesin, phenylephrine hcl liquid
- NDC Code(s): 70000-0629-1
- Packager: CARDINAL HEALTH 110, LLC. DBA LEADER
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
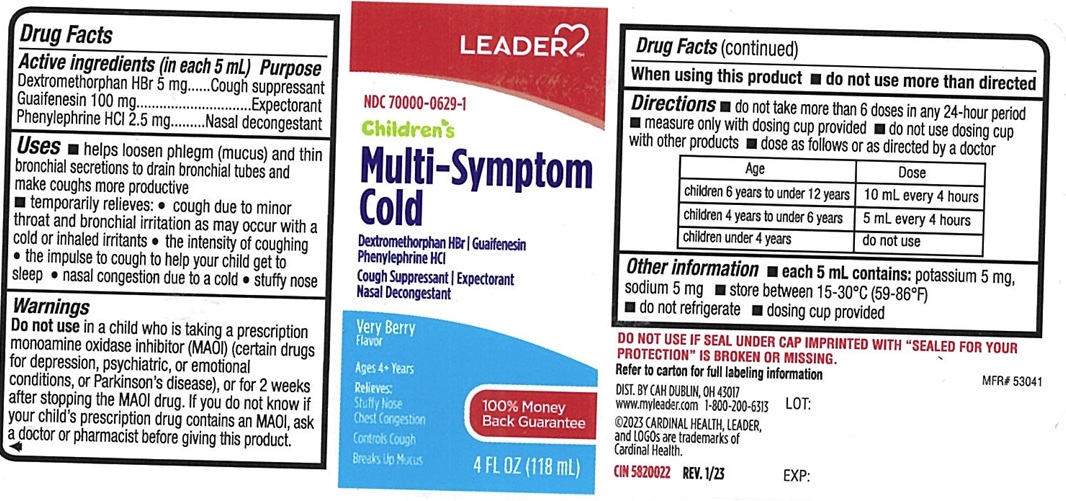
- ACTIVE INGREDIENTS (in each 5 mL)
- PURPOSE
-
USES
- helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes and make coughs more productive
- temporarily relieves:
- cough due to minor throat and bronchial irritation as may occur with a cold or inhaled irritants
- the intensity of coughing
- the impulse to cough to help your child get to sleep
- nasal congestion due to a cold
- stuffy nose
- WARNINGS
-
DO NOT USE
in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not Know if your child's prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
- ASK A DOCTOR BEFORE USE IF THE CHILD HAS
- WHEN USING THIS PRODUCT
- STOP USE AND ASK DOCTOR IF
- KEEP OUT OF REACH OF CHILDREN.
-
DIRECTIONS
- do not take more than 6 doses in any 24-hour period
- measure only with dosing cup provided
- do not use dosing cup with other products
- dose as follows or as directed by a doctor
Age Dose children 6 years to under 12 years 10 mL every 4 hours children 4 years to under 6 years 5 mL every 4 hours children under 4 years do not use - mL=milliliter
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS OR COMMENTS?
-
PRINCIPAL DISPLAY PANEL
LEADER
NDC 70000-0629-1
Children's Multi-Symptom Cold
Dextromethorphan HBr
Guaifenesin
Phenylephrine HCl
Cough Suppressant
Expectorant
Nasal Decongestant
Very Berry Flavor
Ages 4+ Years
Relieves:
Stuffy Nose
Chest Congestion
Controls Cough
Breaks Up Mucus
COMPARE TO
CHILDREN'S MUCINEX®
MULTI-SYMPTOM COLD
active ingredients*
4FL OZ (118 mL)



-
INGREDIENTS AND APPEARANCE
LEADER CHILDRENS MULTI SYMPTOM COLD
dextromethorphan hbr, guaifenesin, phenylephrine hcl liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70000-0629 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 5 mg in 5 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 5 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 2.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) D&C RED NO. 33 (UNII: 9DBA0SBB0L) DEXTROSE (UNII: IY9XDZ35W2) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PROPYL GALLATE (UNII: 8D4SNN7V92) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM ANHYDROUS (UNII: I4807BK602) SODIUM HYDROXIDE (UNII: 55X04QC32I) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color RED Score Shape Size Flavor BERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70000-0629-1 1 in 1 CARTON 03/15/2023 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 03/15/2023 Labeler - CARDINAL HEALTH 110, LLC. DBA LEADER (063997360) Establishment Name Address ID/FEI Business Operations Guardian Drug Company 119210276 MANUFACTURE(70000-0629)


